A Simple Guide to Annual GMP Audit Planning
Still need to plan your 2025 audits? Here's a straightforward guide to doing it the right way.
Since Q3 and Q4 tend to be when we see most RA/QA leaders plan and schedule their annual GxP audits for the coming year, we’re sharing a strategy we’ve seen work for many teams needing to plan audits for multiple sites.
While audit planning shouldn’t be overcomplicated, annual audits across sites deserve a systematic approach that balances risk management with available resources.
This guide provides a structured methodology for QA leaders to develop and implement an effective annual audit program for GMP audits. It’s a loose and adaptable strategy you can use as a starting point to ensure comprehensive coverage of your quality systems while prioritizing areas of most significant risk.
Still need to schedule your 2025 audits? Just a heads up that this year has been the busiest by far for audit planning in recent years. We're seeing RA/QA leaders prioritizing mock inspections, internal site audits, and vendor/supplier audits well into 2025. The FDA is on record saying 2025 will be a “crucial year” for working through its inspection backlog. If you have yet to schedule your audits and other compliance assuredness projects, demand for auditors and mock inspectors — particularly from former FDA professionals — is at an all-time high. We urge you to contact us as soon as possible to make sure resources are available for your audit schedule.
Phase 1: Risk Assessment
The foundation of effective audit planning lies in a thorough risk assessment. This process should typically begin in the third or fourth quarter of the year preceding the audit calendar.
Start by gathering critical data points that will inform your risk evaluation:
Previous audit reports and findings
The status and effectiveness of corrective actions
Regulatory inspection history and outcomes
Current quality metrics and trending data
Process performance indicators
Product complexity and criticality
Recent or planned changes to facilities, processes, or key personnel
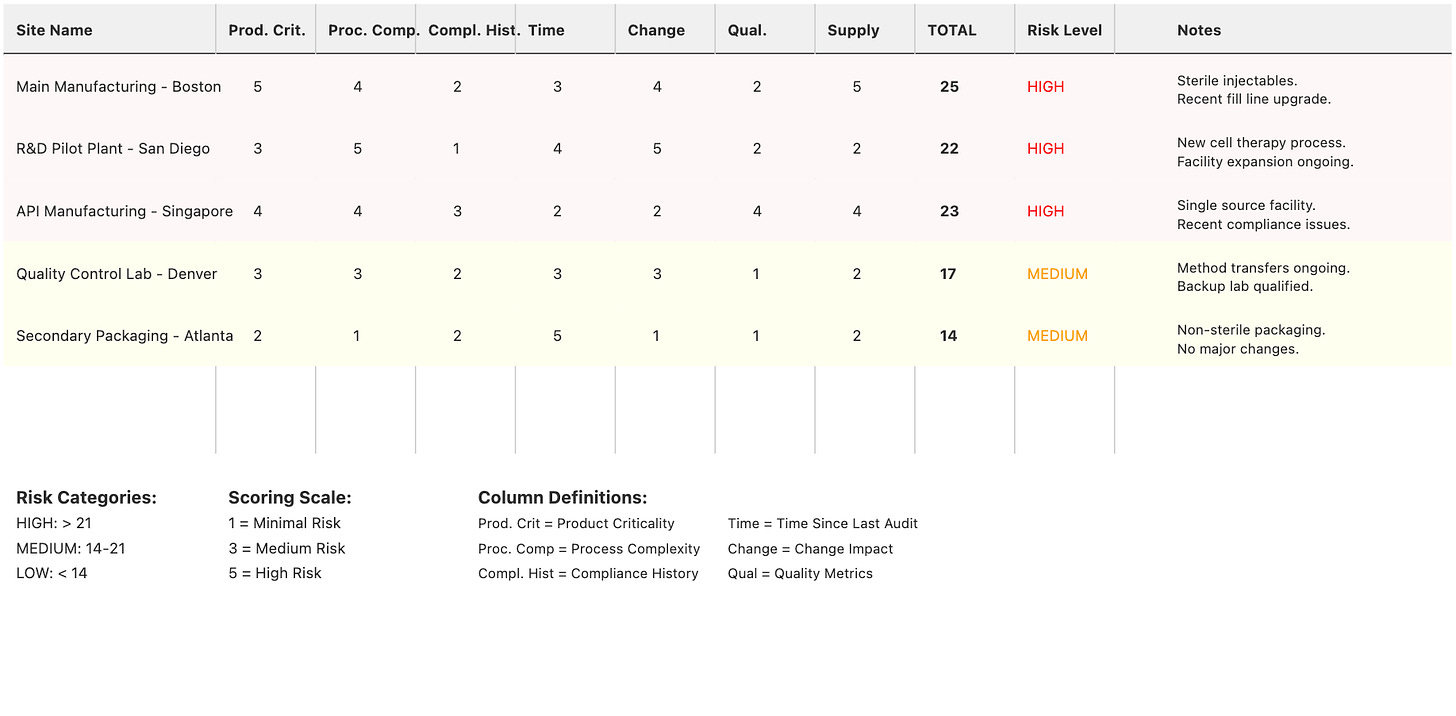
Once this data is collected, develop a simple risk scoring system that quantifies the relative risk of each site or operation.
A simple but effective approach uses a 1-5 scale for each risk factor, where:
Represents minimal risk with robust controls.
Indicates low risk with good historical performance.
Signifies moderate risk requiring normal oversight.
Suggests elevated risk requiring increased attention.
Indicates high risk demanding priority attention.
The factors we suggest folding into this risk assessment for each site include, but of course, shouldn’t be limited to:
Product impact on patient safety
Manufacturing process complexity
Compliance history
Time since the last audit
Change control impact
Quality metrics performance
Supply chain criticality
A simple spreadsheet can serve as a catalog of sites with risk scores and documented risk factors (that of course, may change over time).
Here’s a sample of what a simple living site risk document might look like:
Phase 2: Strategic Planning
With risk scores established, the next phase focuses on developing a strategic framework for the audit program. Consider the following elements:
Audit Types and Scope
Different audit types serve different purposes and require varying resources. Categorize your audits into:
Routine GMP compliance audits
Pre-approval inspection readiness assessments
Supplier qualification/quality audits
Follow-up audits for significant findings
Mock regulatory inspections
For each type, define standard durations and resource requirements. This helps in realistic scheduling and allocating the resources you need for each.
Resource Assessment
Conduct a thorough evaluation of your auditing capabilities or come us with your annual audit plan to get external auditors booked to a schedule.
What expertise and experience will you need for each audit?
Calculate total available audit days, accounting for:
Report writing time
Travel requirements
Other departmental responsibilities
Review budget constraints and travel restrictions
Assess any training or onboarding needs for the audit team
Phase 3: Schedule Development
With risks assessed and resources identified, develop a practical schedule that optimizes coverage while maintaining flexibility.
We always suggest sequencing your audits based on risk scores and operational factors:
Begin with the highest-risk sites early in the year.
Schedule pre-approval inspections to align with regulatory submissions.
Group geographically proximate sites to optimize travel.
Build in contingency time for unexpected events.
Allow adequate preparation and reporting time.
A few practical considerations:
If possible, avoid scheduling audits during peak production periods.
Account for local holidays and site-specific blackout dates.
Allow buffer time between audits for report completion and CAPA initiation.
Phase 4: Communication and Implementation
Clear communication is essential for program success. Develop a structured communication plan for internal and external stakeholders.
Present the annual plan to Quality leadership for approval.
Share the approved schedule with audit team members or anyone else involved.
Distribute to site Quality heads and key stakeholders.
Establish regular update mechanisms.
When communicating with the sites, you’ll be auditing:
Provide formal notification at least 90 days in advance.
Include clear scope and expectations.
Request confirmation of key personnel availability.
Share specific logistics requirements.
Phase 5: Monitoring and Adjustment
A simple monitoring system should be set up to track program effectiveness and make necessary adjustments.
Here’s what we typically suggest here:
Conduct quarterly reviews to assess the completion statuses against the schedule, emerging risk factors, resource utilization, findings trends, and CAPA effectiveness.
Maintain clear records of schedule changes and justifications, audit completion status, key findings and trends, resource utilization, and budget tracking.
A Few Best Practices From the Field
Use simple tools to manage your audit program. Successful audit programs start with straightforward, accessible tools that enable consistent execution. Rather than complex software solutions, focus on maintaining standardized templates in familiar formats like Excel or SharePoint. For risk assessments, create pre-weighted scoring matrices that account for both obvious factors (time since the last audit) and subtle ones like the complexity of material flow between buildings or the number of different regulatory markets served. Build communication templates with specific prompts for sites to provide pre-audit data, such as trending analyses of their top five CAPAs and any open change controls that impact GMP spaces or critical systems.
Flexibility must be engineered into the program systematically. Beyond the standard 20% time buffer for unexpected events, consider implementing a "sister site" system where facilities with similar operations are cross-trained on each other's processes. This creates natural backup coverage options when urgent situations arise. Also, consider creating half-day "rapid assessment" audit protocols that can be deployed quickly when needed, focusing on critical systems and recent changes. These shorter formats help manage the inevitable schedule disruptions while still maintaining oversight.
The most sophisticated audit programs embrace advanced continuous improvement practices. For example, track not just findings but also "near misses" — areas where controls are legally compliant but showing early warning signs of potential issues. We’ve seen really savvy teams build out a "findings forecast" by analyzing trends across similar facilities to predict where problems might emerge next.
Develop site-specific "audit rotation plans" that systematically vary the focus areas examined in depth each time, ensuring comprehensive coverage over multiple visits while preventing audits from becoming routine or predictable.
Also, consider implementing a "reverse audit" program where sites evaluate the effectiveness of the corporate audit approach and suggest improvements based on their experiences being audited.
Pay special attention to auditor development by creating detailed technical guides for each type of operation (sterile, API, packaging, etc.) that go beyond basic requirements to include common failure modes, optimal testing approaches, and interpretation guidance. Track which auditors excel at identifying different types of issues and use this data to optimize team compositions.
Maintain a library of exemplary responses to common findings that demonstrate the expected level of detail and systemic thinking in corrective actions.
Watch our interviews with Divya Gowdar and former FDA investigator Chris Smith for more insights into auditing and inspection readiness.
Need 2025 audit support support? Let’s talk.
Need rapid access to the industry’s top auditors? Contact us to access our exclusive pool of 2,500+ global consultants, 255+ of whom are former FDA.
If we haven't yet partnered on project support, we provide end-to-end audit support and intensive, comprehensive mock FDA inspections that not only mirror real inspections but go deeper — giving you the insights, corrections, and readiness you need before the FDA walks through your doors.
With a staff of thousands of resources worldwide, we're the partner firms work with when they want deep domain expertise and the peace of mind that comes with a partner whose commitment to quality and integrity reflects their own.
Whether you need a complete audit program design, full execution, or specific audit support, we're here to help. Drop us a line to start the conversation.
A few links and resources
Who is The FDA Group?
The FDA Group helps life science organizations rapidly access the industry's best consultants, contractors, and candidates. Our resources assist in every stage of the product lifecycle, from clinical development to commercialization, with a focus on Quality Assurance, Regulatory Affairs, and Clinical Operations.
With thousands of resources worldwide, hundreds of whom are former FDA, we meet your precise resourcing needs through a fast, convenient talent selection process supported by a Total Quality Guarantee. Learn more and schedule a call with us to see if we’re a fit to help you access specialized professionals and execute your projects on time and on budget.