[Bonus Issue] Top 5-Year Trending FDA GMP Inspection Citations (FY2018 to FY2022)
Wondering which GMP-related CFRs FDA is citing most frequently? Check out our interactive data visualizations and prioritize your compliance assurance activities.
This is a free bonus issue for all subscribers. Only paid subscribers can access our regular monthly newsletters.
Each year, we update our long-running report on the top trends that emerge from FDA’s public inspection and enforcement action datasets available from the agency’s data dashboard.
The report provides a quick look at the ten most frequently cited CFRs related to GMP in both the drug and device spaces over the past five fiscal years.
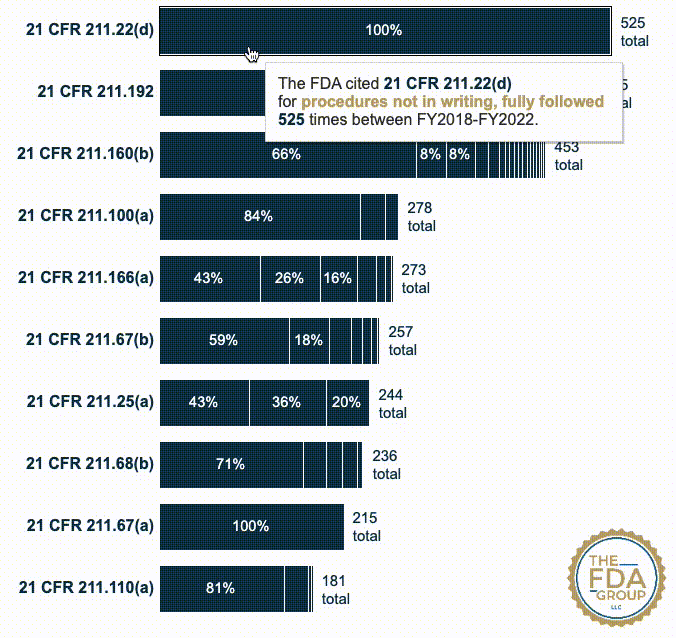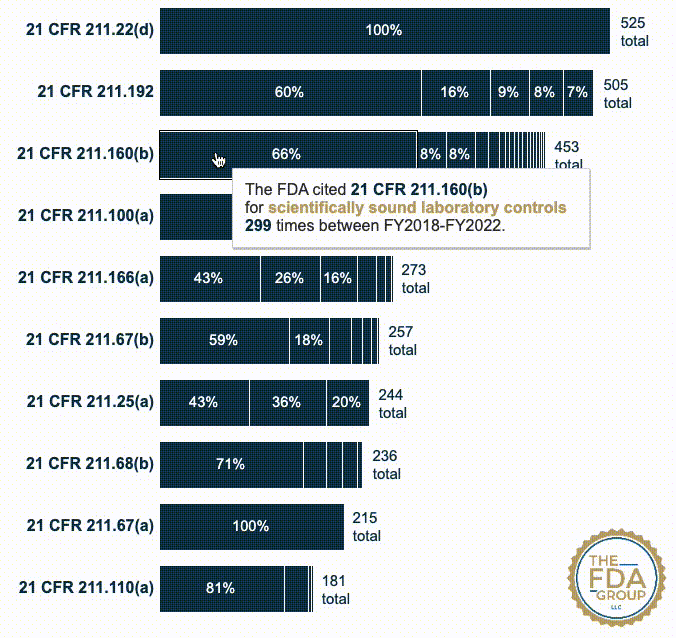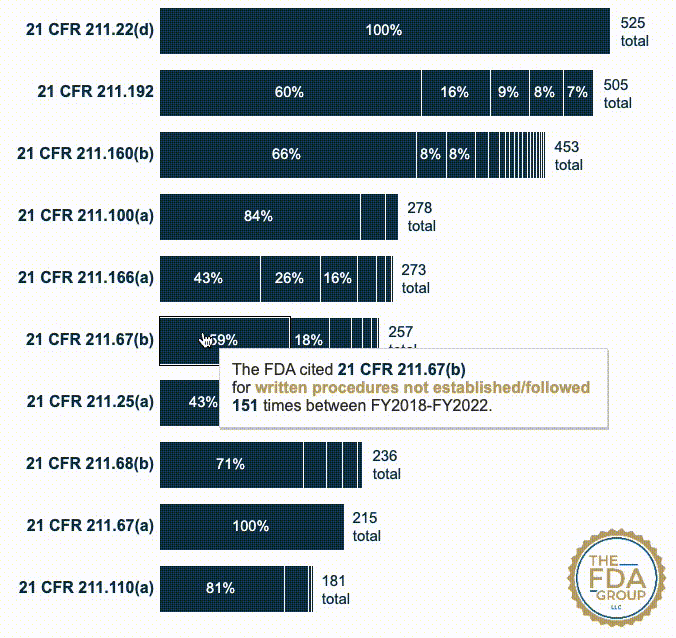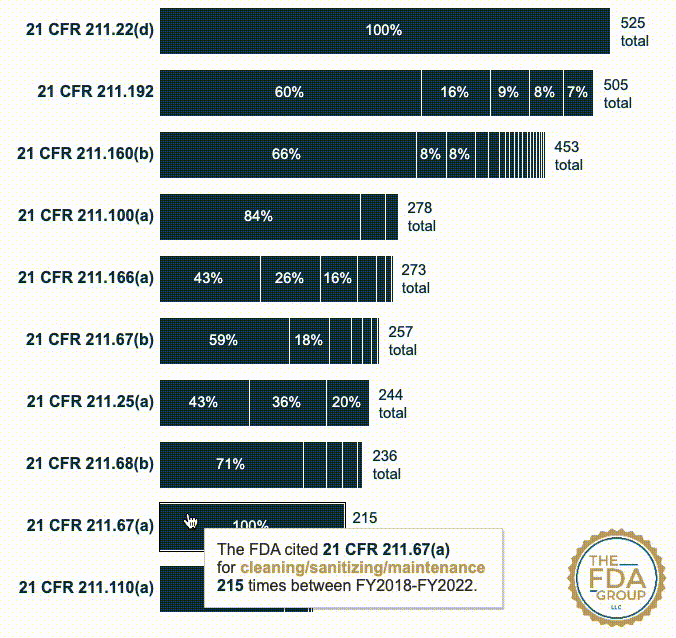
In this year’s report, which includes cumulative data from FY2018 to FY2022, we added two interactive graphics that break those CFRs down by the various “short descriptions” FDA associates with each inspection citation. These descriptions provide useful context for seeing which specific problem(s) FDA is citing within a given CFR or subpart.
Here’s a snippet from FDA’s inspection citations dataset to illustrate what information the agency logs:
Our new interactive data visualizations show the percentage of each short description under each top-cited CFR—also distinguishing specific subparts when they’re broken out in the data.
Since Substack doesn’t allow us to embed the visualizations themselves, the animated gif below shows how it works. Again, you can view and interact with these visualizations in our report.
Here are links to open each visualization directly from Tableau: Drug | Device.
Top 5-Year GMP Inspection Citations
Here are simple lists of the top ten trending GMP inspection citations for drugs and devices.
Note: Citations data include Form FDA 483 citations from FY2018 to FY2022 and may not necessarily represent citations on final classification letters. Read FDA’s data caveats on its data dashboard page.
Drug
21 CFR 211.22(d) — The responsibilities and procedures applicable to the quality control unit shall be in writing; such written procedures shall be followed (525 citations)
21 CFR 211.192 — Production record review (505 citations)
21 CFR 211.160(b) — Laboratory controls shall include the establishment of scientifically sound and appropriate specifications, standards, sampling plans, and test procedures... (453 citations)
21 CFR 211.100(a) — There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess... (278 citations)
21 CFR 211.166(a) — There shall be a written testing program designed to assess the stability characteristics of drug products. The results of such stability testing shall be used in determining appropriate storage conditions and expiration dates... (273 citations)
21 CFR 211.67(b) — Written procedures shall be established and followed for cleaning and maintenance of equipment, including utensils, used in the manufacture, processing, packing, or holding of a drug product... (257 citations)
21 CFR 211.25(a) — Each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform the assigned functions... (244 citations)
21 CFR 211.68(b) — Appropriate controls shall be exercised over computer or related systems to assure that changes in master production and control records or other records are instituted only by authorized personnel... (236 citations)
21 CFR 211.67(a) — Equipment and utensils shall be cleaned, maintained, and, as appropriate for the nature of the drug, sanitized and/or sterilized at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements. (215 citations)
21 CFR 211.110(a) — To assure batch uniformity and integrity of drug products, written procedures shall be established and followed that describe the in-process controls, and tests, or examinations to be conducted on appropriate samples of in-process materials of each batch. Such control procedures shall be established to monitor the output and to validate the performance of those manufacturing processes that may be responsible for causing variability in the characteristics of in-process material and the drug product... (181 citations)
Medical Device
21 CFR 820.100(a) — Each manufacturer shall establish and maintain procedures for implementing corrective and preventive action (923 citations)
21 CFR 820.198(a) — Each manufacturer shall maintain complaint files. Each manufacturer shall establish and maintain procedures for receiving, reviewing, and evaluating complaints by a formally designated unit... (790 citations)
21 CFR 820.50 — Each manufacturer shall establish and maintain procedures to ensure that all purchased or otherwise received product and services conform to specified requirements. (394 citations)
21 CFR 820.90(a) — Each manufacturer shall establish and maintain procedures to control product that does not conform to specified requirements... (393 citations)
21 CFR 803.17 — If you are a user facility, importer, or manufacturer, you must develop, maintain, and implement written MDR procedures... (382 citations)
21 CFR 820.22 — Each manufacturer shall establish procedures for quality audits and conduct such audits to assure that the quality system is in compliance with the established... (381 citations)
21 CFR 820.75(a) — Where the results of a process cannot be fully verified by subsequent inspection and test, the process shall be validated with a high degree of assurance and approved according to established procedures... (371 citations)
21 CFR 820.30(g) — Design validation. Each manufacturer shall establish and maintain procedures for validating the device design... (348 citations)
21 CFR 820.184 — Each manufacturer shall maintain device history records (DHRs)... (345 citations)
21 CFR 820.50(a) — Evaluation of suppliers, contractors, and consultants. Each manufacturer shall establish and maintain the requirements, including quality requirements, that must be met by suppliers, contractors, and consultants... (245 citations)
Companion webinar: Identifying and Preventing the Most Common Drug and Device cGMP Issues
In 2021, we hosted a free, two-hour webinar event with accomplished RA/QA consultant Neal Siegel, who addressed many of the top trending drug and device citations year to year and offered firsthand insight into proactive motions teams should consider to ensure compliance. It’s a great companion resource to this data that offers some practical guidance on how to detect and address common problems in each of these CFRs.
The FDA Group helps life science organizations rapidly access the industry's best consultants, contractors, and candidates.
Need expert help planning and executing compliance projects or connecting with a quality and compliance professional? Get in touch with us. If we haven’t worked together yet, watch our explainer video below or head to our company introduction page to see if we could help you execute your projects now or in the future.
Our service areas:
Quality Assurance | Regulatory Affairs | Clinical Operations | Commissioning, Qualification, and Validation | Manufacturing and Engineering | Expert Witness
Our engagement models:
Consulting Projects | Staff Augmentation | FTE Recruitment
Our podcast:
Apple | Spotify | YouTube | Web + Others