FDA's CBER Revises its SOPP for Processing and Reviewing INDs: What Sponsors Need to Know
A quick breakdown of CBER's recent SOPP revisions for incoming INDs.
This guidance breakdown is available in full for paid subscribers. If you’re not already a paid subscriber, you can upgrade here.
Editor’s note (7/16/2024): We have updated section #1 below with clarification from CBER.
The SOPP 8217 document specifically states: "CBER staff will not terminate an inactive IND that has been inactive for 5 years or more if the inactive IND is already being cross-referenced."
Our interpretation that this update explicitly allows sponsors to reference data from inactive INDs in new or active IND submissions was an inference that is not directly supported by the text of the SOPP.
For those involved in the development and clinical investigation of new drugs and biologics, the FDA's CBER revised its SOPP 8217 on July 5th regarding how it processes and reviews INDs, the application type used to seek clearance to run clinical trials.
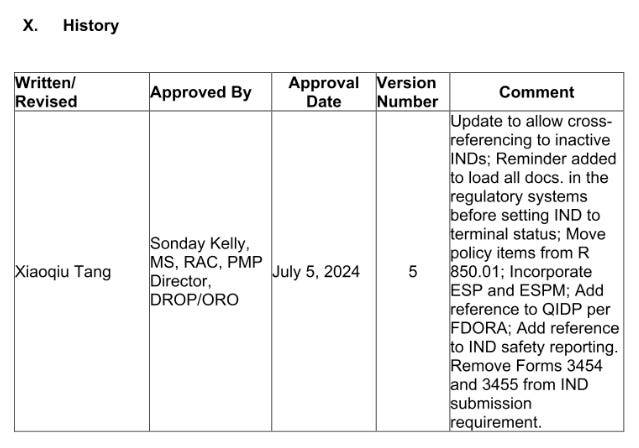
Here’s the excerpt of the change record capturing the change notes:
We’ve quickly broken each of these updates down, along with some recommendations for sponsors in light of them where action might be warranted.
1. Protecting cross-referenced inactive INDs being cross-referenced from termination
Update from CBER:
Following some confusion over the interpretation of this update, we reached out to CBER for clarification and received the following reply from the Division Director, Sonday L. Kelly (we’ve updated this section accordingly):
The update was a clarification that INDs in terminal status may not be cross-referenced. The revision table mentions inactive INDs as an additional point of clarification - inactive INDs are not in terminal status, thus may be cross-referenced to support other regulatory submissions.
The edit you have below ["CBER staff will not terminate an inactive IND that has been inactive for 5 years or more if the inactive IND is being cross-referenced"] is part of the change to the SOPP and serves to ensure FDA staff prevent cross-referenced, inactive INDs from going into a terminal status, i.e., termination.
We have also updated the following in Procedures, Step 8d:
Ensure that the cross-referenced INDs are not in terminal status. If cross-referenced INDs are in terminal status, notify the sponsor that terminated INDs may not be cross-referenced, because the sponsor of a terminated IND has no obligation to submit reports or to update the file.
The update to SOPP 8217 to "allow cross-referencing to inactive INDs" seems to refer to the policy outlined in Section V:
"CBER staff will not terminate an inactive IND that has been inactive for 5 years or more if the inactive IND is already being cross-referenced."
The SOPP doesn't appear to be showing any new policy explicitly allowing cross-referencing to inactive INDs; it would have been more accurate to say something like "Update to protect cross-referenced inactive INDs from termination" or "Clarification on handling of cross-referenced inactive INDs."
Instead, it shows a policy of not terminating inactive INDs that are already being cross-referenced.
The most notable implication is that — as the SOPP states — inactive INDs that are being cross-referenced will not be terminated by CBER, even if they've been inactive for 5 years or more. This provides some assurance to sponsors that valuable data in inactive INDs will remain accessible if it's being used in other applications.
Also, CBER updated its procedures, Step 8d:
Ensure that the cross-referenced INDs are not in terminal status. If cross-referenced INDs are in terminal status, notify the sponsor that terminated INDs may not be cross-referenced, because the sponsor of a terminated IND has no obligation to submit reports or to update the file.
Here, the office is instructing its staff to ensure INDs aren’t cross-referencing terminal INDs and to notify the sponsor if this is the case.
2. Reminder to load all docs into regulatory systems before setting an IND to “terminal” status
This appears to be an internal reminder that before an IND is designated as terminal (i.e., no longer active and not intended to be reactivated), all relevant documents must be uploaded to the FDA’s regulatory systems.
3. Move policy items from R 850.01
Certain policy items previously located in Regulatory Reference R 850.01 have been moved to SOPP 8217.
Firms should review the updated SOPP 8217 to familiarize themselves with the relocated policy items and ensure they are following the latest procedural guidelines. Regulatory Affairs teams will want to make sure they’re referencing moved policies in this document instead of R 850.01. Update your internal regulatory manuals, SOPs, and training materials to reflect the new location of these policies.
4. Incorporate ESP and ESPM
Incorporation of the Electronic Submission Program (ESP) and Electronic Submission Program Manager (ESPM) roles and responsibilities into the SOPP.
Firms should align their submission processes with the ESP's requirements and guidelines and ensure that they engage with the ESPM as necessary for submission validation and troubleshooting. For instance, if a submission encounters technical issues, the ESPM can assist in resolving these to ensure timely and compliant submissions.
Firms may want to assign a team member to engage regularly with the ESPM for guidance and troubleshooting. Also, consider providing technical training for staff on the use of the ESP system and addressing common validation issues.