GAO: FDA Should Implement Strategies to Retain Its Inspection Workforce
A new U.S. Government Accountability Office report finds persistent challenges within the FDA’s drug inspection program, which continues to struggle with reduced capacity and inexperience.
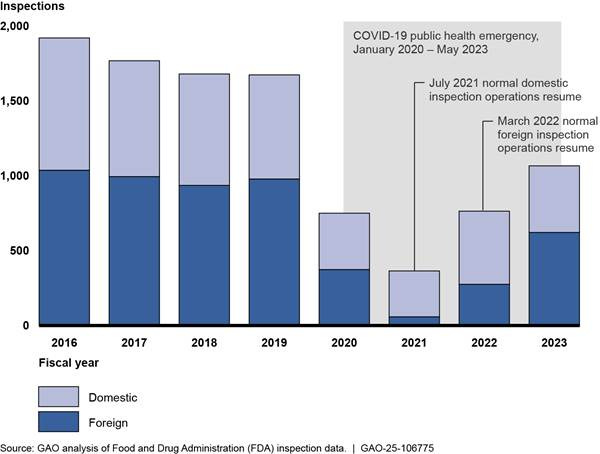
The U.S. Government Accountability Office (GAO) is out with a new report outlining concerns regarding the staffing of the FDA’s drug inspection program. The report highlights that the agency is conducting significantly fewer inspections than its peak in 2016.
Despite a partial recovery from the backlog of pharmaceutical manufacturing site inspections caused by the pandemic, the GAO reported that in 2023, the FDA conducted 36% fewer inspections than it did in 2019. Also, the number of vacancies for FDA drug investigators increased from 25 (out of approximately 250 total inspectors) in November 2021 to 73 vacancies in 2023.
The FDA has been focused on addressing inspector vacancies that arose during the pandemic. But, the GAO reports that since then, the loss of experienced investigators has outpaced hiring efforts, leading to a significant number of relatively inexperienced inspectors. This situation has limited the FDA's capacity to conduct inspections.
The GAO identified several main causes for this issue: the frequency and conditions of travel, inadequate pay, insufficient training, heavy workloads, and challenges related to work-life balance, though it says the FDA is currently taking steps to improve pay and training to address these concerns.
By contrast, the GAO said that by 2023, as routine surveillance inspections increased following the pandemic, the percentage of inspections classified as “official action indicated” — the worst designation a site can receive following an inspection — “decreased and was similar to pre-pandemic rates.”
Despite the lingering staffing issues, the GAO said the FDA has continued to rely on trusted foreign regulators’ inspection work in lieu of conducting its own on-site inspections, while the use of remote assessments “has declined and will largely be reserved for more targeted use now that inspections have resumed.”
The GAO report indicated that increasing globalization and a rise in foreign inspections of the drug supply chain have posed challenges for the agency’s investigative workforce. As a result of attrition, the agency now has a less experienced workforce and reduced capacity to conduct necessary inspections.
The report urged FDA Commissioner Robert Califf to develop plans to address attrition stemming from problems related to investigator travel, workload, and work-life balance.
Here are our key takeaways that offer a deeper look at GAO’s report.
1. The FDA’s inspection capacity has declined post-COVID
The COVID-19 pandemic disrupted the FDA’s inspection operations, particularly for overseas facilities. By fiscal year 2023, the FDA had increased its inspection activities compared to 2022, but inspection totals remained significantly below pre-pandemic levels. According to GAO, the FDA conducted 36% fewer inspections in 2023 than in 2019. This reduction is largely attributed to a limited workforce of trained investigators.
The pandemic backlog in inspections created a critical issue for the FDA's surveillance program, particularly impacting risk-driven surveillance intended to ensure that facilities meet Good Manufacturing Practices (GMP). During the pandemic, the FDA prioritized mission-critical inspections, often for cause or pre-approval, while deferring routine surveillance inspections. Though the FDA has resumed a more routine inspection cadence, the pandemic-era backlog and resulting resource constraints are still affecting the FDA’s ability to meet its inspection goals, particularly for high-risk sites.
2. Investigator attrition and staffing challenges are significant problems
A major contributing factor to the FDA’s reduced inspection capacity is the increase in inspector vacancies since 2021. The GAO report shows that the FDA experienced a rise in vacancies among drug investigators, jumping from 25 in November 2021 to 51 by mid-2024.
These shortages were especially noticeable among general inspectors who handle both domestic and international inspections, as well as among specialists within the foreign cadre.
The GAO report attributes high turnover rates to several factors: