Inside the FDA's Plans to Reform its Human Foods Program and Transform ORA into the Office of Inspections and Investigations (OII)
The FDA is advancing its reorg proposal for a unified human foods program, field operations and other modernization efforts.
This is a bonus issue available to all subscribers. If you’re not already a paid subscriber, you can upgrade here.
On December 13th, senior FDA officials announced the agency's plans to revamp its human foods program and restructure its Office of Regulatory Affairs (ORA) by transforming it into the Office of Inspections and Investigations (OII).
If you haven’t read the FDA’s original news release, we suggest starting there.
The FDA’s reorg package at a glance
As explained in its news release, the FDA's proposed reorganization package includes several key updates to improve the functioning of the agency:
The Health Fraud and Product Centers (HFP) will be solely responsible for receiving, triaging, and closing consumer and whistleblower complaints. This centralizes the complaint process, enhancing the FDA's ability to detect problems more quickly.
The Office of Regulatory Affairs (ORA) will be renamed the Office of Inspections and Investigations (OII), emphasizing its role in field-based inspections, investigations, and import operations.
An Office of the Chief Medical Officer (OCMO) will be established within the Office of the Commissioner to coordinate medical issues across the agency, including those affecting special populations. Additionally, a new Office of Public Health Preparedness and Response will be created to handle medical countermeasure policy, emergency preparedness, and medical product shortage coordination.
The Office of Counterterrorism and Emerging Threats (OCET) and the Office of Regulatory Science and Innovation (ORSI) will merge into a new office in the Office of the Chief Scientist (OCS), named the Office of Regulatory and Emerging Science, to bolster regulatory science and preparedness research.
An Office of Enterprise Transformation will be created to drive business process improvement across the FDA.
Before these changes are implemented, the FDA states they must undergo several steps, including review by HHS, the Office of Management and Budget, a 30-day notification period to Congress, a Federal Register notice, and negotiations with Unions representing affected staff.
The FDA’s reorg webinar
A few days later on December 19th, the Alliance for a Stronger FDA hosted a webinar where several FDA officials dug deeper to explain the reorg plans — an initiative they say is in part a response to criticism from Congress of its handling of the 2022 infant formula crisis.
The Alliance’s webinar featured Principal Deputy Commissioner Janet Woodcock, Chief Scientist Namandjé Bumpus, Associate Commissioner for Regulatory Affairs Michael Rogers, Deputy Commissioner for Human Food Jim Jones, and Donald Prater, acting director of the Center for Food Safety and Applied Nutrition (CFSAN).
Watch the webinar recording below.
Woodcock, who is departing from the agency next month, mentioned that the reorganization proposal has been delayed due to internal bureaucracy:
“We're really trying to transform different aspects of the FDA to make them more efficient, more effective, and so forth … Our mission seems to continually be broadening and we really do need to be able to meet our mission as much as our resources allow.”
But officials are optimistic that the proposal will be implemented this year once there’s an agreement between the FDA's unions. Additionally, she stated that one of the objectives of the proposal is to prevent duplication of functions at ORA. The plan is budget-neutral.
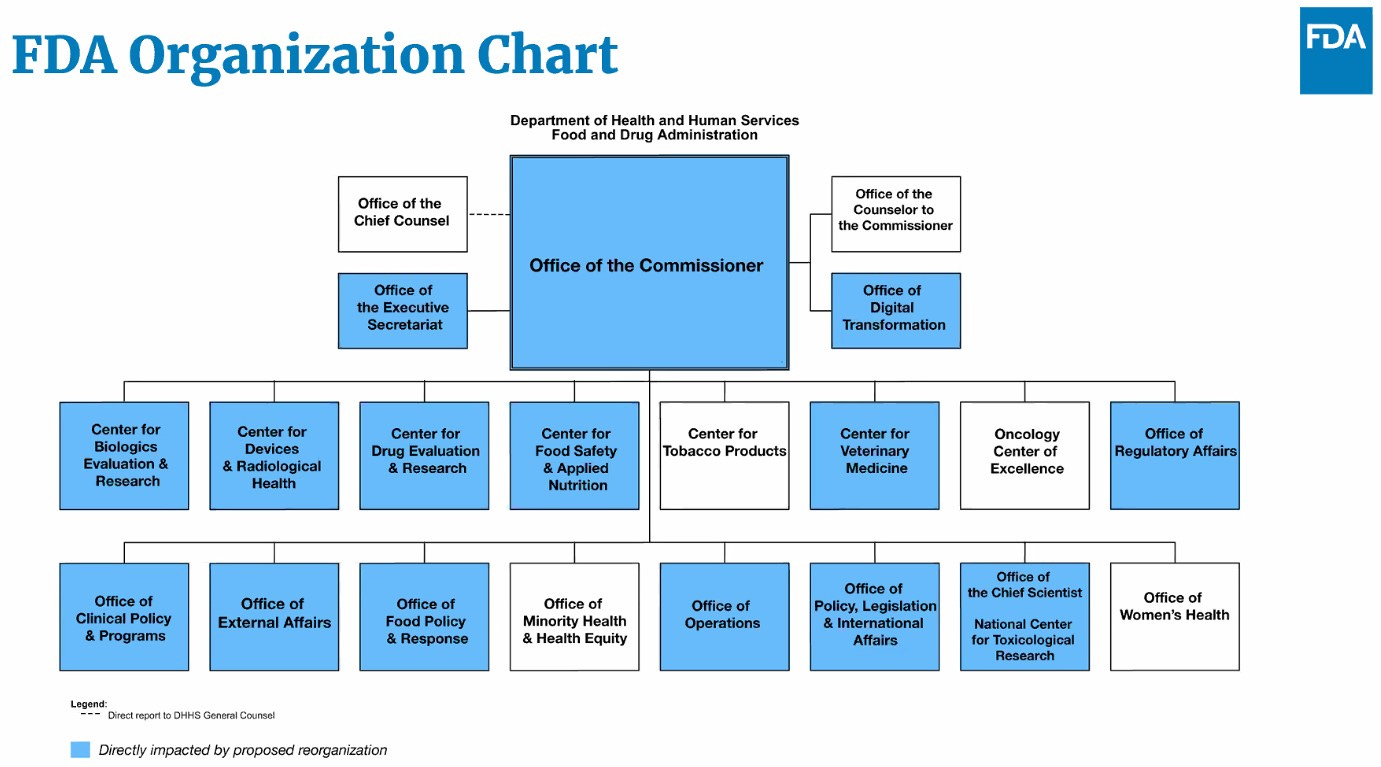
In the webinar, Woodcock mentioned that the FDA submitted their reorganization plans to HHS last month. She also stated that the agency will be more transparent about how its budget is utilized in the future.
She also shared an organizational chart illustrating that most FDA offices will be impacted in some way by the reorganization.
Michael Rogers, Associate Commissioner for Regulatory Affairs, discussed the transformation of ORA into OII, which will concentrate on inspections, investigations, and import operations. Compliance functions, except for imports, will be transferred to established product centers and compliance programs.
“That alignment will eliminate a lot of duplication and bring investigators closer to the center program staff, especially when the agency needs to react to and evaluate an ongoing violative inspection … In addition, most consumer complaint responsibilities in ORA, specifically intake and evaluation, and closeout, will be realigned to the human foods program and the centers, and OII will carry out any necessary field evaluation for complaints when requested by the human foods program in the centers and will also retain a small team of complaint personnel to make sure these field evaluations are conducted in a streamlined manner.”
As a result of the alignment, Rogers noted ORA staff would shrink from about 5,100 to 3,600 at OII. The remaining 1,500 staff would be reassigned to the agency’s product centers.
Rogers proposed that the reorganization would lead to better tracking of product complaints throughout their lifecycle, ensure that complaints are handled promptly, and that serious complaints are escalated to senior leadership for resolution. The human food laboratories will be assigned to the human foods program, while other product laboratories will be assigned to the Office of Chief Scientist.
He also presented a chart showing how OII will operate, adding that there will be several cross-cutting functions that it will be responsible for.
“For example, our Office of Criminal Investigations remains essentially unchanged and will carry out criminal investigation activities for all of the FDA-regulated products ... We’ll have an Office of Field Operations and Response that will provide enterprise inspectorate support, including organizational quality efforts and within it there'll be a division of tobacco inspectorate to conduct tobacco inspectional work.”
Rogers also announced that his office will establish an Office of Emergency Response to manage the FDA's response to emergencies and natural disasters related to FDA-regulated products and facilities — excluding foodborne outbreaks, which will be managed by the human food program. Previously, this responsibility was under the Office of Operations.
Chief Scientist Namandjé Bumpus restated the plan’s review process the agency laid out in its initial news release, explaining that the proposed reorganization plan will undergo a review process by the HHS. Once this is completed, the plan will be sent to the Office of Management and Budget (OMB) for further review. Congress will then be notified and given a 30-day period to comment on the plan. A Federal Register notice will also be published to allow stakeholders to give their feedback. Finally, the FDA will start negotiations with staff unions.
She added that the FDA is “hopeful that implementation will occur sometime in calendar year 2024.”
Now that the agency has submitted its reorganization plans to HHS, Woodcock says that the agency is currently figuring out the specifics to make sure the transition occurs without any issues. She stated that there might be an initial period of adjustment as employees relocate and work with slightly different methods, but believes that the transition will go smoothly if the reorganization is implemented this year.
A few potential implications for industry
The reorganization of ORA into OII, and the reallocation of staff to product centers, could have several important implications for companies regulated by the FDA that are worth considering:
Potentially more efficient inspections and investigations. With ORA staff reassigned to focus more directly on inspections and investigations, FDA-regulated companies might actually experience more efficient and possibly more frequent inspections. This change could lead to quicker resolution of compliance issues but also require companies to be more vigilant in maintaining standards and compliance assuredness.
A more specialized office (and investigators). The reassignment of staff to product centers could result in inspectors and investigators who are more specialized in specific product areas. This might lead to more thorough and knowledgeable inspections and evaluations, requiring companies to have a deeper understanding of regulations specific to their products and be able to communicate more fluently about the technical nuances of their products and operations.
Potentially streamlined compliance and enforcement processes. The reorg aims to eliminate duplicative functions within the ORA. While we’re being optimistic, this could potentially lead to a more streamlined and efficient compliance and enforcement process. We know firsthand that industry would like to see clearer communication and faster decision-making from the FDA. Here’s to being hopeful.
A need to adapt to the new org structure. Companies will need to adapt to the new structure of the FDA, including understanding the roles of the new OII and adjusting to new points of contact within the reorganized agency. We have a few specific suggestions here:
Invest some time in understanding the specific functions and responsibilities of the OII. Anticipate how interactions with this new office might differ from those with the previous ORA structure.
As staff are reassigned and new roles are established, it’ll be important for companies to identify their new points of contact within the FDA. Building relationships with these contacts can facilitate smoother communication and more effective resolution of any regulatory issues.
With the change in the FDA's structure, there might be new or modified compliance expectations. Companies should review and update their internal protocols to align with these changes.
Train your regulatory affairs and quality assurance staff on the new FDA structure and any new procedures or requirements that come with it. This training should include understanding the nuances of interacting with the OII.
Assess how the reorganization might affect any pending applications with the FDA and plan for potential impacts on future submissions.
Understanding that the OII will focus specifically on inspections and investigations, companies should prepare for potential changes in these areas, possibly including more specialized inspections.
Additional resources
Who is The FDA Group?
The FDA Group helps life science organizations rapidly access the industry's best consultants, contractors, and candidates. Our resources assist in every stage of the product lifecycle, from clinical development to commercialization, with a focus on Quality Assurance, Regulatory Affairs, and Clinical Operations.
With over 2,500 resources worldwide, over 225 of whom are former FDA, we meet your precise resourcing needs through a fast, convenient talent selection process supported by a Total Quality Guarantee.