Preparing for the QMSR: A Comprehensive Guide + Gap Analysis Worksheet
Paid subscribers can download our free QMSR white paper and gap analysis worksheet.
The medical device industry is on the cusp of a significant regulatory shift as the FDA harmonizes the QSR with ISO 13486:2016. Is your organization ready?
Our new white paper and gap analysis worksheet are your essential guides for transitioning from the FDA's QSR to the new Quality Management System Regulation (QMSR).
Paid subscribers get:
A Deep Dive into QMSR: Understand the key changes, their implications, and how they align with ISO 13485:2016.
Transition Timeline and Strategy: Get a clear roadmap for compliance, including critical deadlines and actionable steps.
Risk Management Focus: Learn how to integrate robust risk management practices throughout your quality system.
Inspection Readiness: Prepare for the new inspection landscape under QMSR.
Gap Analysis Guidance: Identify and address discrepancies between your current QMS and QMSR requirements.
Checklist: Get our exclusive QMSR Transition Checklist to track your progress and ensure no critical steps are missed.
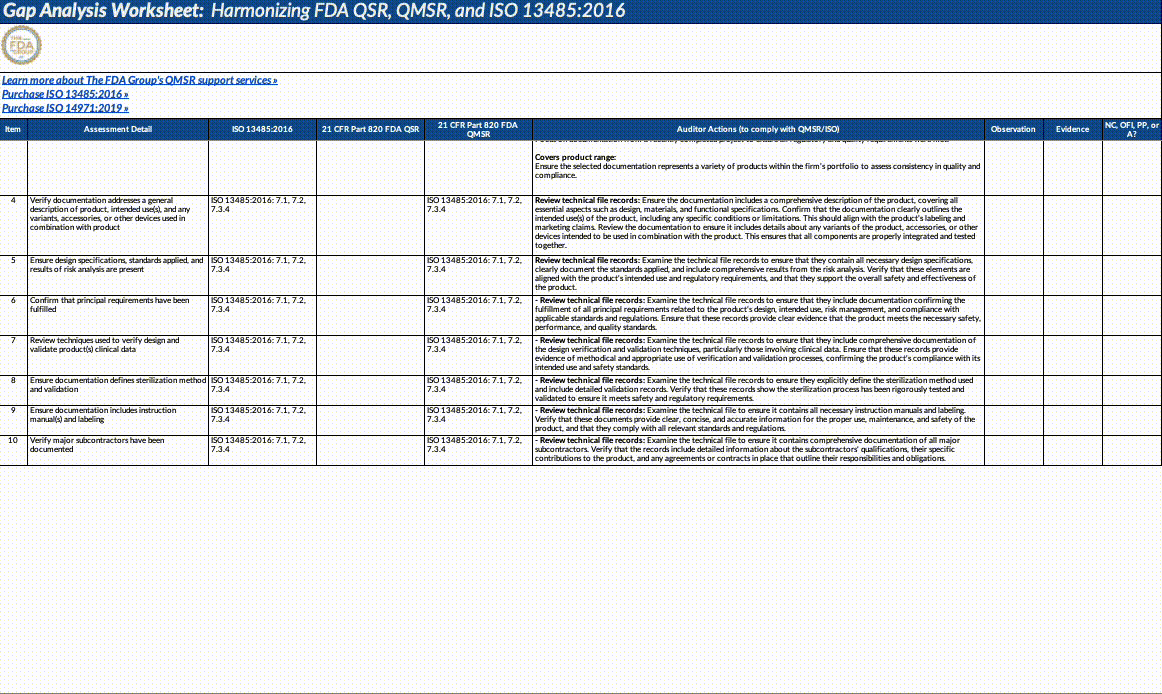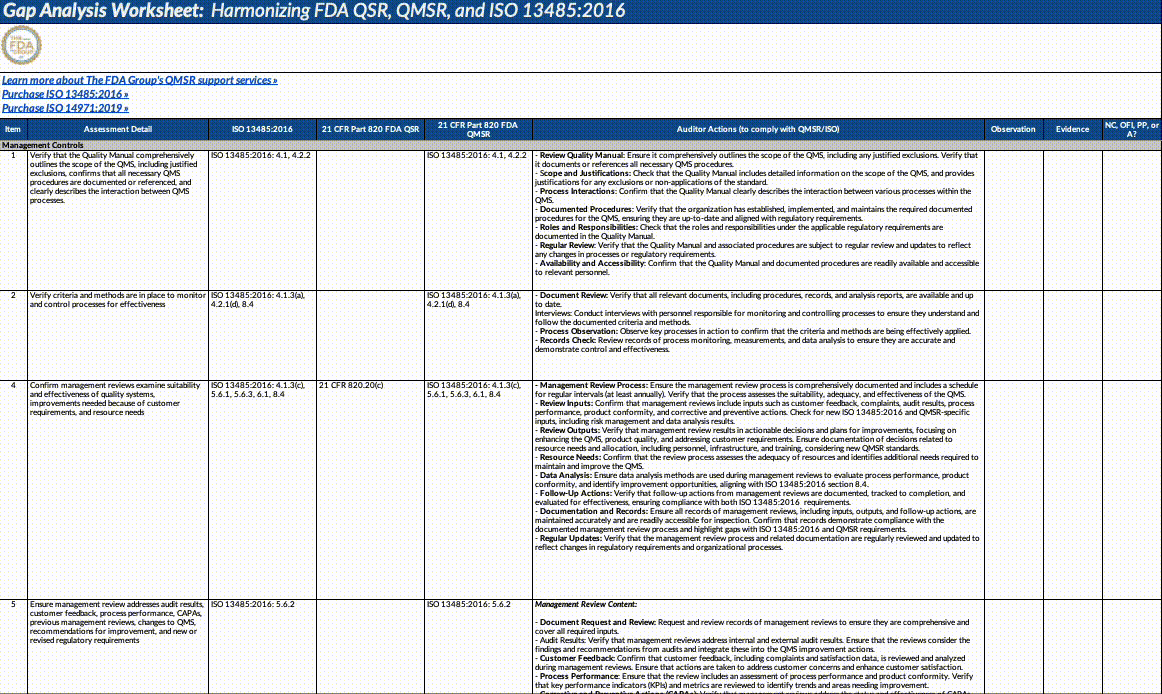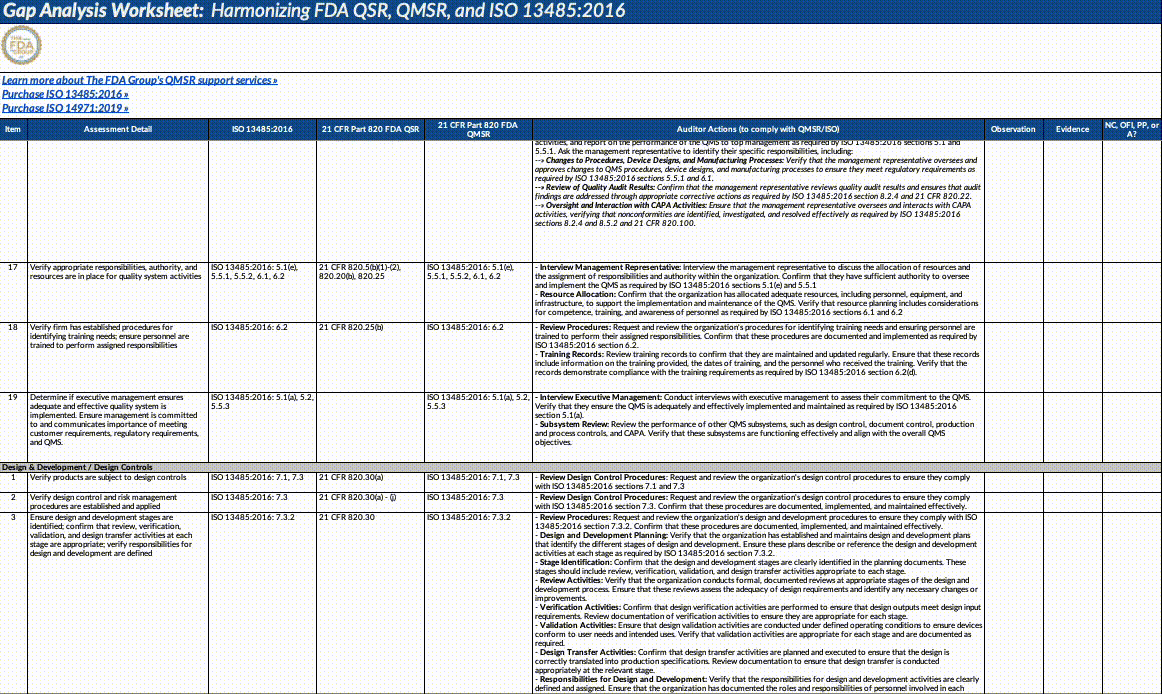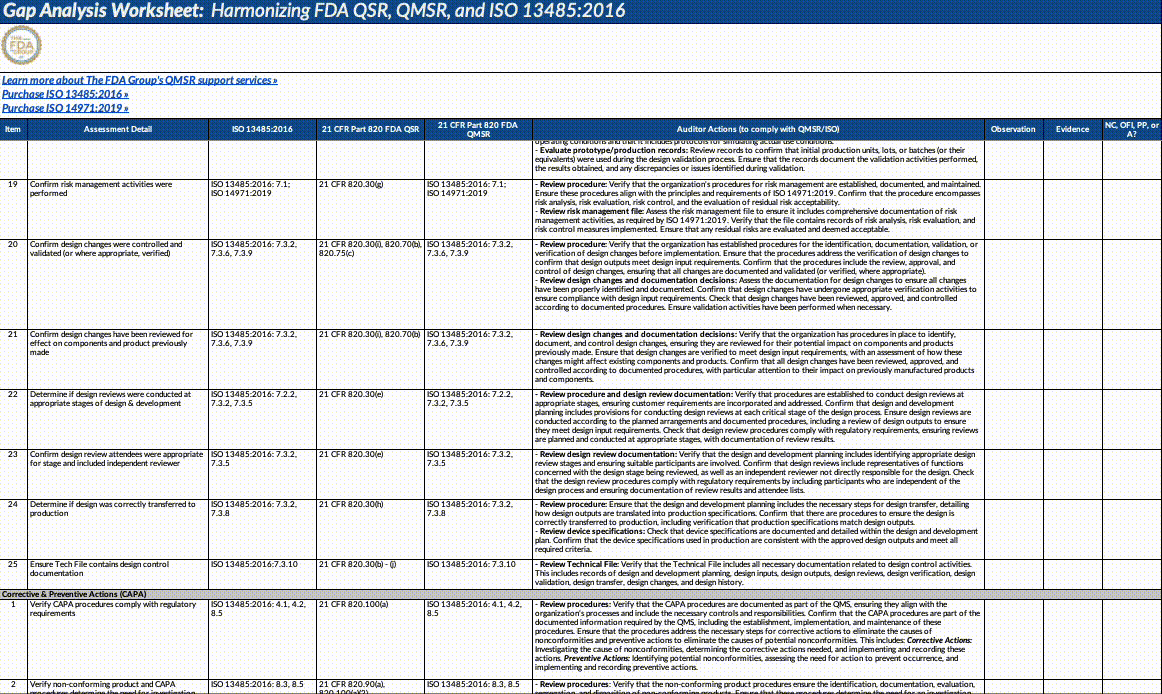
Gap Analysis Worksheet Structure:
Assessment Detail: Detailed descriptions of what needs to be assessed, including specific auditor actions, document reviews, and employee interviews.
Auditor Actions: Specific steps auditors should take to verify compliance with QMSR/ISO requirements.
Observation: Space to record observations made during the audit or assessment.
Evidence: Documentation or evidence that supports the observations.
Outcome (NC, OFI, PP, A): The result of the assessment, categorized as NC: Non-Conformance, OFI: Opportunity for Improvement, PP: Positive Practice, A: Adequate/Acceptable
Whether you're ISO 13485 certified or primarily QSR compliant, this white paper provides invaluable insights to ensure a smooth transition to QMSR.
Don't let the February 2026 deadline catch you off guard. Download these tools now and take the first step towards QMSR compliance.
The links to both of these resources are below the paywall here. Graduate to our paid tier to access them.