The Anatomy of an Effective Supplier Audit Report
We break down our own supplier audit template.
This article is only available in full to paid subscribers. If you’re not already a paid subscriber, you can upgrade here.
Since Q3 and Q4 tend to be when we see most RA/QA leaders plan and schedule their annual supplier audits for the coming year, we’re sharing our own supplier audit report template.
We walk you through the key components of our reports, providing context, insights, and best practices for each section, along with clarifying examples from an actual sample report.
Still need to schedule next year’s supplier audits? It’s annual audit planning season and we’re scheduling audits for teams into 2025. Our certified, experienced auditors plan, schedule, and execute vendor/supplier quality management audits to identify areas of conformance and nonconformance with applicable global regulations. Following assessment, our experts provide a detailed report including all observations, deficiencies, and a risk-based corrective action plan for improvement. Learn more about our supplier audit services.
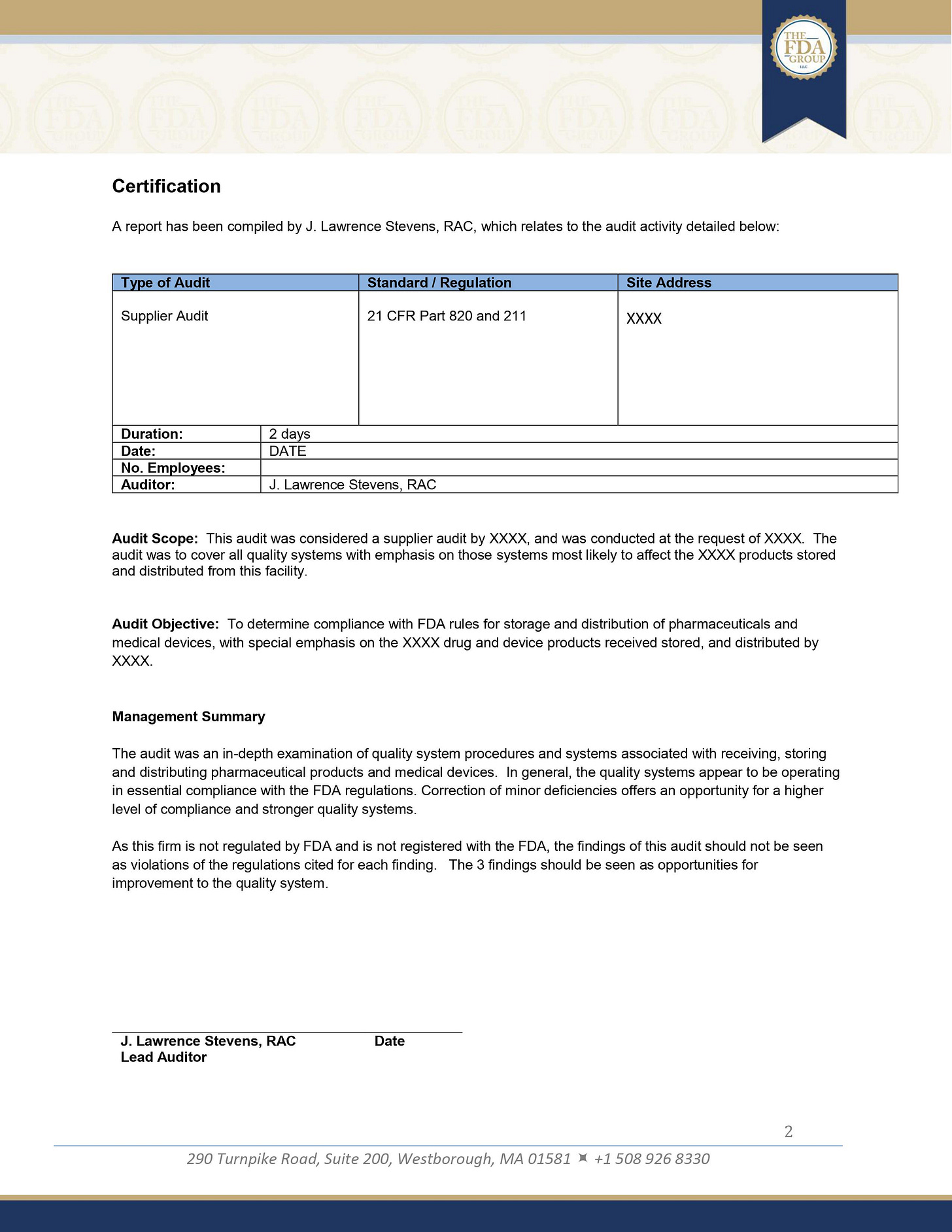
1. Title Page
The title page serves as the first impression of your audit report. It should be professional, clear, and contain all the essential information at a glance.
The title should be specific, e.g., "Supplier Audit Report for [Supplier Name]" rather than just "Audit Report."
Including the auditor's qualifications (such as RAC - Regulatory Affairs Certified) demonstrates the credibility and expertise of the person conducting the audit.
The date on the title page should reflect when the audit was conducted, not when the report was written, to provide context for the findings.
2. Certification Statement
This section acts as a formal declaration of the report's authenticity and the auditor's involvement.
The certification statement serves a legal purpose, establishing the auditor's responsibility for the report's contents. It should be concise but comprehensive, covering the critical aspects of the audit activity. This statement can be crucial if the report is ever used in legal proceedings or regulatory inspections.
3. Audit Details
This section provides the essential context for understanding the scope and nature of the audit.
Specifying the type of audit (e.g., Supplier Audit) helps set expectations for what the report will cover. Citing the relevant regulations (e.g., 21 CFR Part 820 and 211) demonstrates that the audit was conducted against recognized standards.
Including the duration of the audit and the number of employees indicates the depth and complexity of the assessment. This information helps readers understand the context of the findings and recommendations.
4. Audit Scope and Objective
Clearly defining the scope and objective is crucial for framing the entire audit report.
The scope should be specific and bounded. For example, "all quality systems with emphasis on those systems most likely to affect the [Client] products" gives a clear focus.
The objective should be aligned with regulatory requirements and the client's needs. For instance, "To determine compliance with FDA rules for storing and distributing pharmaceuticals and medical devices" sets a clear purpose.
This section helps manage expectations about what the audit does and does not cover, preventing misunderstandings later.
5. Management Summary
The management summary provides a high-level overview of the audit findings for busy executives or stakeholders who may not read the entire report. This summary often informs high-level decision-making, so it should be written with care and precision.
This section should be concise but comprehensive, highlighting the most significant findings and their implications. It's important to provide context for the findings. For example, noting that the firm is not FDA-registered helps frame the recommendations appropriately.
The tone should be balanced, acknowledging both strengths and areas for improvement.
6. Key Functions and Key Personnel
This section introduces the main actors involved in the audit process and the supplier's operations.