Guidance Breakdown: Cross-Center Master Files – Where to Submit
What MF holders, sponsors, and combination-product teams need to know about the FDA’s new cross-center hosting recommendations.
This guidance breakdown is available in full to paid subscribers. Only paid subscribers get regular full access to our guidance breakdowns and other analyses. If you’re not already a paid subscriber, you can upgrade here.
Welcome back from the holiday if you’re stateside. Today’s breakdown takes us into a niche but increasingly important corner of FDA practice: master files that need to be used across more than one center.
The FDA just issued the draft guidance “Cross-Center Master Files: Where to Submit.” As the title suggests, it doesn’t change what goes into a master file or how it’s reviewed. Instead, it provides center-level recommendations on where to submit master files that will be referenced by more than one FDA center, especially in combination-product and other “cross-center” scenarios.
The draft, developed by the Office of Combination Products with CBER, CDER, CDRH, and CVM, is aimed at a familiar pain point for many RA and CMC teams: uncertainty over which center should “host” a master file when multiple centers will ultimately need to access it. FDA reiterates that master files are voluntary and discourages duplicate or “convenience copy” files in multiple centers to preserve version control, noting that any center can access a properly referenced master file regardless of where it resides.
In this quick breakdown, we walk through what the guidance actually says, who it applies to, how the FDA defines “cross-center” master files, the decision framework for selecting a hosting center, and how the Agency expects holders and sponsors to think about combination products, human vs. animal drugs, and shared platforms like container-closure systems.
A quick refresher on key MF concepts
The draft formalizes a few definitions you’ll want to align with your internal vocabulary:
Master File (MF) – A voluntary submission that provides confidential, detailed information (e.g., facilities, processes, articles used in manufacturing/packaging/storage, nonclinical data, shared system REMS) that can support one or more regulated products. FDA reviews MFs only in the context of referencing submissions; they are never “approved” on their own.
Master File Holder – The organization or person that submits the MF and can authorize others to reference it.
Letter of Authorization (LOA) – A letter from the MF holder allowing a sponsor/applicant/other MF holder to incorporate part or all of the MF by reference and authorizing FDA to use it in support of that party’s submission. It does not allow the authorized party to see the MF’s contents.
Referencing Submission – Any regulatory submission (or another MF) that cites an MF. Examples include INDs, NDAs, ANDAs, BLAs, PMAs, 510(k)s, de novo requests, animal drug applications, and IDEs.
Lead Center – The center with primary review responsibility for the referencing submission (e.g., CDER-led drug–device combo).
Hosting Center – The center where the MF is located in FDA’s systems.
Cross-Center Master File – An MF that will be accessed by more than one center to support one or more referencing submissions.
The guidance also reiterates a few foundational points about MFs:
MF use is voluntary and at the holder’s discretion; MFs are not required by regulation.
FDA discourages duplicate submissions of the same MF to multiple centers (including “convenience copies”) to preserve version control.
Once an MF is in FDA’s systems, any center can access it when properly referenced, regardless of where it’s hosted.
From a sponsor/holder standpoint, that last point is key: you don’t need separate MFs in multiple centers to support cross-center use. But you do need to pick a hosting center that lines up with the FDA’s recommendations.
Why the FDA cares about “where” your MF lives
Cross-center use of MFs is already common. The FDA gives several examples where more than one center needs to access a single MF to complete its review:
A CDER-led drug–device combination product (e.g., drug-filled autoinjector) references a CDER MF describing the container closure system/device constituent part. CDRH accesses the MF via a consult on the device portion.
A CDRH-led drug–device combination product (e.g., drug-eluting stent) references a CDER MF describing the drug coating. CDER accesses the MF via a consult on the drug portion.
A container-closure-system MF is used in CBER, CDER, and CVM submissions; all three centers access the file for their respective products.
A component MF (e.g., drug substance or excipient) is used both in human drugs (CDER) and animal drugs (CVM).
An MF describing facilities or manufacturing processes for drugs is referenced in both CBER and CDER drug submissions (e.g., NDAs).
Historically, MF holders have taken different approaches, sometimes sending the MF to the center of the lead application, sometimes to the center they thought was “closest” to the MF’s subject matter. FDA notes this has created uncertainty and inefficiency.
The new recommendations aim to standardize which center should host MFs that will be used across centers and reduce duplicate submissions and the version-control issues that come with them. For your internal teams, that should translate into fewer “where do we send this?” debates, clearer expectations from the FDA, and less risk that an MF is fragmented across centers.
The FDA’s core decision framework: three screening questions
When you’re deciding where to submit an MF, the FDA asks holders to consider three things.
1. What is the purpose of the MF information?
Is it proprietary design or testing information for a specific constituent part of a combination product? Is it broader information (e.g., a facility, container closure system, component) that may support many products?
2. Which center will receive the referencing submission?
CBER? CDER? CDRH? CVM? Are multiple centers expected to receive referencing submissions over time?
3. Will the MF support a combination product submission?
If yes, what is the lead center and which constituent part is covered in the MF?
The FDA then splits its detailed recommendations into two buckets: combination-product scenarios and non-combination-product scenarios. If, after applying this framework, you still cannot determine the hosting center, the FDA explicitly invites MF holders to engage the Agency to resolve it.
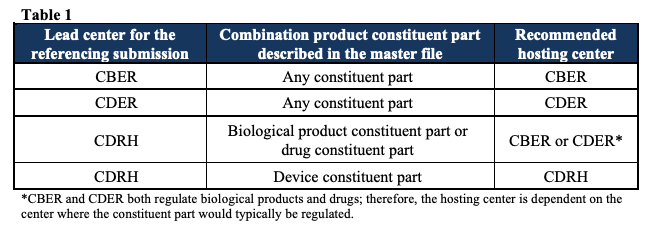
Combination products: follow the lead center, then the constituent part
For combination products, the recommendation is simple in intent: start with the lead center and the specific constituent part your MF describes. The FDA recommends that MFs referenced by combination-product submissions “typically include information about only one constituent part” of the combination product.
The hosting rules then fall out as follows:
General combination-product rules
CBER-led combination product: If the MF contains information about any constituent part (biologic, drug, or device) of a CBER-led combination product →
Submit the MF to CBER.CDER-led combination product: If the MF contains information about any constituent part (drug, biologic, or device) of a CDER-led combination product → Submit the MF to CDER.
CDRH-led combination product: If the MF contains information about the device constituent part → Submit the MF to CDRH. If the MF contains information about the drug or biological product constituent part, submit it to CBER or CDER, depending on where that constituent part would be regulated as a standalone product.
Table 1 in the guidance summarizes this logic.
Special case: constituent parts marketed under separate applications
The guidance calls out a less common but important scenario:
a combination product whose constituent parts are each marketed under their own applications in different centers.
One example given is a product composed of a drug constituent part marketed under an NDA, and a device constituent part marketed under a PMA. In this case, an MF that supports the drug constituent part and is referenced by the NDA → Submit to the center receiving the NDA. An MF that supports the device constituent part and is referenced by the PMA → Submit to the center receiving the PMA.
The operational takeaway here: For combination products, don’t over-optimize by trying to guess which center is “most technical” for your content. Once you’ve identified the lead center and which constituent part your MF is about, the hosting center is effectively determined by this table.
For non-combination products, route to the center receiving the submission
When the MF supports non-combination products, FDA’s default recommendation is:
The hosting center should be the center that will receive the referencing submission.
However, the guidance recognizes that some MFs naturally span more than one center even outside combination-product scenarios (e.g., an inactive ingredient used in both human and animal products). It addresses these.
If a single MF will be referenced by non-combination submissions in more than one center, the recommendation is to submit the MF to the center that will receive the first referencing submission. The FDA suggests coordination between MF holders and the applicants/sponsors to determine which center is likely to receive that first referencing submission, so you can route the MF appropriately.