Stability Across the Lifecycle: The FDA-ICH Draft Q1 Guideline Explained
The FDA just released an extensive draft guidance document on drug stability testing that was first published earlier this year by the ICH. We break down this 100+ page draft.
This guidance breakdown is available in full to paid subscribers. Only paid subscribers get regular full access to our guidance breakdowns and other analyses. If you’re not already a paid subscriber, you can upgrade here.
Back in April, the ICH endorsed a consolidated draft, “Stability Testing of Drug Substances and Drug Products (Q1),” and yesterday, the FDA issued it for U.S. comment.
The document is the ICH-endorsed consolidation of the entire legacy Q1A–F series and Q5C—meaning one global standard now governs stability for synthetic APIs, biologics, vaccines, gene & cell therapies, conjugates, ATMPs, drug–device combinations, and co-packaged diluents (radiopharmaceuticals and medical-device components remain out of scope).
When final, it will anchor every U.S. stability submission, from IND-enabling batches through lifecycle changes. Comments on the draft are open until August 25.
While there’s no simple way to distill a massive guidance like this, we’ve broken it down at a high level, if nothing else, to assist your own reading of it.
How to read and operationalize this guideline
Parsing and acting on a 100+ page technical guideline is not easy. Here’s a plan to slice through it strategically with your team in a sort of playbook.
You don’t need to read these pages linearly. Triage the guideline into bite-sized ownership blocks tied to the roles that will actually execute the work. One concentrated morning of focused, role-based reading turns a 100-page behemoth into a clear action plan you can operationalize long before the ink dries on the final Q1.
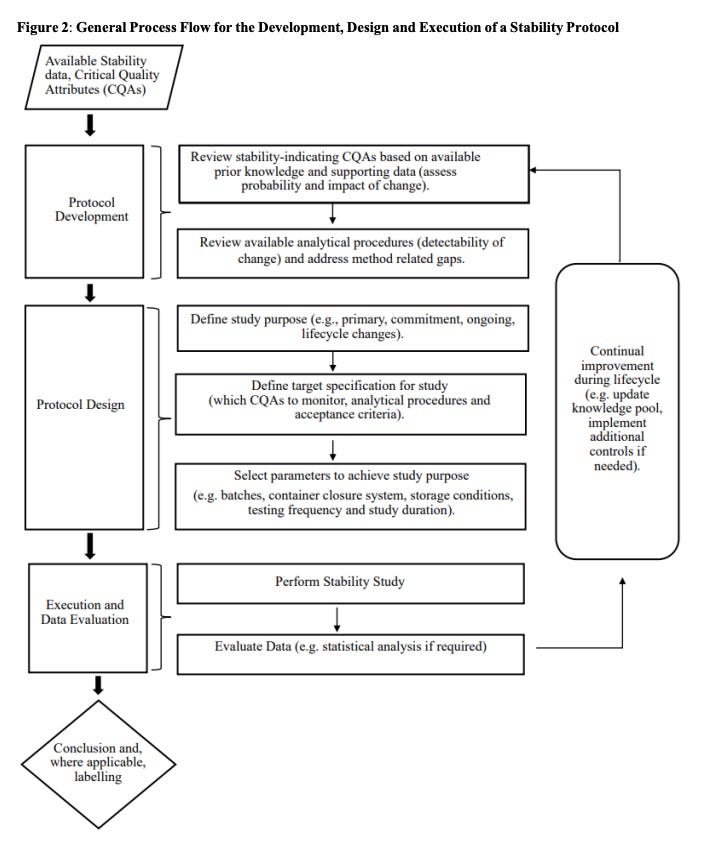
Read §1–§3 front-to-back. They define scope, new stress/forced degradation expectations, and the stepwise protocol flow (Fig. 2) that drives everything that follows. Make sure your QA Lead and CMC Project Manager are versed in it. Then, write up which of your legacy protocols already align and which don’t.
Jump to §7 “Storage Conditions” + Table 3. Decide now whether you will commit to Zone IVb 30°C/75% RH or stick with 25°C/60% RH, and understand the four mitigation paths if you fail the severe zone. From this, you can write up a go/no-go slide on adopting Zone IVb and list out your products needing pilot stress runs.
Scan §4 “Batch Selection” & §5 “Container/Closure.” Verify the three-batch rule, the comparability requirement for biologics, and the new quantitative data expected to justify bracketing/matrixing. Then, draft a simple matrix indicating which products already have three representative batches versus which require new lots.
Read §13 + Annex 2 together. These pages dictate statistical pooling tests, regression rules, and extrapolation limits—often the section FDA reviewers cite in complete-response letters! Whoever manages your stability stats should draw up a stats “charter” template: slope/intercept tests, α-levels, software, and a simulation plan.
Scan the Annex 1 decision trees. See when bracketing, matrixing, or reduced test frequency are acceptable, and the penalties if the design is too aggressive. Take this and build a checklist to embed in a protocol template so teams must justify each reduction.
Review §14 “Excursions & Labelling.” Make sure you understand the new 24-hour excursion trigger and how stress-study data can support field-excursion statements. You might need to tweak your SOP: data-logger alarm at 18h, along with an excursion assessment form.
Finish with §15 “Lifecycle Stability.” This section clarifies commitment, ongoing, and change-support programs—and ties them to Q12 PAC-MP logic. Have whoever coordinates your change controls draw up a flowchart linking every PAC code to a stability protocol type.
As you go through this guideline, assign roles first, pages second. Give each SME a slice they alone own (table above). Collect their summaries into a single slide deck—now everyone learns from everyone else. Teams that divvy up projects like this often hold a 45-minute “stability stand-up.” Each owner presents their one-pager in five minutes. The deck becomes your gap-action register.
We also suggest starting a living cross-reference sheet. One column = Q1 section number; next = SOP number or protocol ID that addresses it; blank cells = gaps. Pilot the high-risk clauses first. Run a small-scale Zone IVb study and a full forced-degradation panel on a top-seller to uncover surprises before the guideline is final.
Alright, let’s break this down.
Objectives and scope
The guideline’s purpose is to show “how the quality of a drug substance or drug product varies with time” under temperature, humidity, light, agitation and other stresses so that a scientifically justified re-test period or shelf life can be assigned.
Recommendations apply across all climatic zones (I–IVb) and embrace the Quality-by-Design toolbox in Q8–Q12 and Q14. Importantly, where the text says “products,” read “drug substances and drug products.”
Development studies: stress vs. forced degradation
Stress studies use conditions more severe than the accelerated tier (e.g., > 40 °C, thermal cycling, freeze-thaw) but do not deliberately degrade the material. One batch each of drug product (and, if needed, drug substance) suffices. Data can help justify label-claim excursion tolerances later.
Forced-degradation studies deliberately attack the molecule—75 % RH or higher, wide pH, oxidation, photolysis, agitation/heat combos—to map degradation pathways, confirm stability-indicating methods (tie-in to Q2/Q14), and support control-strategy design. One batch of drug substance (synthetics) or drug product (biologics) is recommended, and testing stops once “extensive decomposition” occurs.
Results of both study types funnel into selecting critical quality attributes (CQAs) and long-term test methods.
Formal stability protocol design
A step-wise flow (Figure 2) walks sponsors from product knowledge to protocol:
Standard dataset—three primary batches, 12 months long-term + 6 months accelerated for NCEs; abbreviated 6 mo/6 mo set for generics; biologics supply three primary & production batches with ≥ 6 months data at filing.
Stability-indicating CQAs—include potency, purity/impurities, physico-chemical attributes, microbiology, and any device-function metrics (Section 3.3).
Risk management—science- and risk-based protocol adjustments are encouraged and should be justified (Section 3.7).
Batch selection
The guideline calls for choosing three representative primary batches manufactured by processes “comparable” to commercial scale (pilot acceptable with justification). If multiple sites appear in the initial dossier, site-specific data can be risk-adjusted, but full programs are still due post-approval (Section 4.2).
Continuous-manufacturing campaigns need batch definitions aligned to residence-time concepts (Section 4.4).
Container-closure and testing frequency
Section 5 emphasises matching orientation, material, and secondary packaging between stability and commercial packs. Characteristics such as surface-area-to-volume ratio, water-vapour and oxygen-permeation rates, and stopper formulation guide “extreme” selections for reduced designs.
Testing frequency (Section 6) follows legacy Q1A expectations unless a justified reduction is applied.
Storage conditions and climatic zones
Table 3 in Section 7 harmonizes long-term, intermediate, and accelerated settings per zone:
Zones I–II: 25 °C ± 2 °C/60 % RH ± 5 % (long-term) → 40 °C/75 % RH (accelerated).
Zone IVb: 30 °C ± 2 °C/75 % RH ± 5 % (long-term) → 40 °C/75 % RH (accelerated).
Running at the most severe zone (30 °C/75% RH) can support worldwide labeling, but failure under that regimen forces one of four mitigation paths (e.g., shorter shelf life, alternative container). Excursions > 24h outside tolerances must be recorded and their impact assessed.
Impermeable containers: humidity studies optional. Any RH condition is acceptable (Section 7.2.1).
Semi-permeable containers: relative-humidity selection may leverage permeation-coefficient calculations. Example 1 in Section 7.2.2 shows the math for Zone III products.
Photostability program
Two big components in Section 8:
Forced photodegradation—exposure to light “more extreme” than confirmatory testing to gauge intrinsic photosensitivity (Section 8.2).
Confirmatory studies—run under ICH Q1B Option 1 or 2 conditions to verify label protection claims (Section 8.3).
Light-source requirements and minimum lux hours are specified in Section 8.4.
Data evaluation & shelf-life modelling (Section 13 + Annex 2)
The guideline is clear that linear regression of individual batches is the default. Proposed shelf life must be no longer than the shortest single-batch estimate unless a statistical test justifies pooling.
Combining batches—prospective statistics should test slope & intercept similarity before pooling; simulation studies are encouraged.
Scale transformation and non-linear kinetics—log or other transforms may give a worst-case shelf life when degradation decelerates; non-linear regression is acceptable with justification.
Extrapolation—extension beyond measured data is allowed for synthetics and, under defined conditions, biologics (Annex 2).
Reduced designs—Annex 1 in action
Two concepts here to dig into:
Bracketing—test only the extremes of strength and container size. Table A1-1 shows a 3-strength/3-size liquid with 15 mL and 500 mL extremes, each in triplicate batches, at every time-point. Design risks include the “least-stable extreme” rule—intermediate packs cannot claim longer shelf life than the worst extreme.
Matrixing—rotate subsets across time-points. Separate matrix per storage condition and never matrix across different test attributes. Design factors cover multiple strengths, container sizes, foil overwraps, and biologic concentration variants.
Special topics snapshot
A few other things to note:
Vaccines and biologics—require extra potency checks. Accelerated data confirms method specificity rather than assigning shelf life (Section 3.5; Section 7 note).
Combination products—overall shelf life cannot exceed the shortest constituent. Assembled units must be tested (Section 3.6).
Processing and holding times—Section 9 introduces stability considerations for intermediates awaiting downstream steps.
A few action items we recommend for putting Q1 into practice
Here are detailed steps we recommend teams consider to operationalize this guidance:
1. Re-map every stability document to the new Q1 structure.
We suggest most teams start here by creating a cross-reference matrix. List all your legacy protocols written to Q1A-F/Q5C headings and map them to the nearest Q1 sections (e.g., old “Q1A 2.2 Stress Testing” now falls under Section 2.2 Development Studies Under Forced Degradation).
Two other things come to mind here:
Gap-hunt for missing narratives. Q1 explicitly wants rationales for each stress and forced-degradation condition—humidity ≥ 75% RH, pH extremes, oxidation, heat/light combos, and “stop when extensive decomposition occurs.” Make sure to insert a short justification in every protocol explaining why each condition is relevant to your molecule and dosage form.