The Economics of Quality—Why FDA Says It’s Time to Double-Down on a Mature QMS
A four-stage cost curve shows exactly where your QMS investment begins to compound returns. We break down the FDA's latest white paper.
This is a special free edition of our Insider Newsletter. Only paid subscribers get regular full access to our guidance breakdowns and other analyses. If you’re not already a paid subscriber, you can upgrade here.
At the of July, the Center for Drug Evaluation and Research’s Office of Pharmaceutical Quality (OPQ) released Quality Management Initiatives in the Pharmaceutical Industry: An Economic Perspective—a 21-page white paper that sets out, in plain economic terms, why quality management is no longer just a compliance obligation but an actual profit lever and a public-health safeguard.
It really is a must-read for anyone in the life sciences, but especially QA professionals and those who hold their budgets.
The document is split into two distinct arguments:
Part I quantifies the economic return on incremental and step-change investments in quality.
Part II frames the public-health dividend that flows from a more resilient, shortage-proof supply chain.
Here’s a quick breakdown with some thoughts from us.
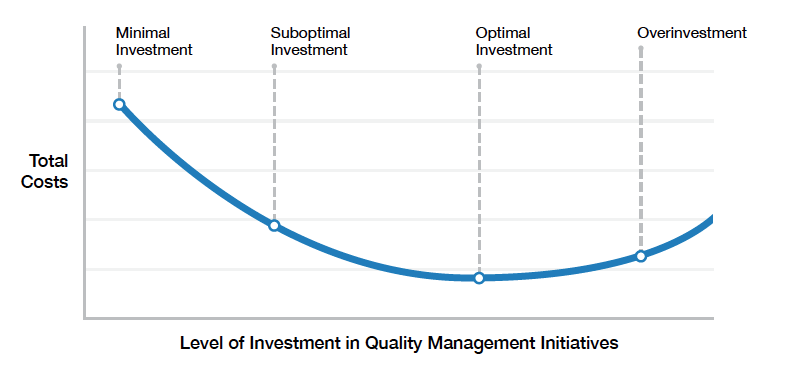
The cost curve that should be on every COO’s wall
Borrowing a classic "cost-of-quality" framework, the FDA maps four investment scenarios—minimal, sub-optimal, optimal, and over-investment—onto a single cost curve supported by microeconomic principles.
The white paper presents the mathematical relationship:
TC = F + V + sQ - f(Q)F - g(Q)V
Fixed and variable costs (F and V) represent baseline operational expenses.
sQ represents the level of investment in quality management initiatives.
f(Q)F and g(Q)V represent the savings generated from those investments.
Up to the "optimal" point, every additional dollar spent on quality drives costs down through efficiency gains and defect reduction. Beyond that, diminishing returns set in. It's a reasonable model that we see get reflected in real life all the time.
Crucially, the agency reminds the industry that moving along this curve "is not an all-or-nothing proposition."
"Incremental investments in quality management initiatives can yield returns. Whether a company is beginning its quality management journey or working its way toward the optimal point, each investment provides the opportunity for additional returns."
Proof in practice—three case studies FDA highlights
The paper lays out a few illustrative case studies that put some proof points on the claims.
Culture-first basics beat defects. At one biopharma site sitting at "intermediate" maturity, leadership doubled down on culture and core process discipline. The FDA reports: "This site reduced product defects by more than 50% and waste by 75%; additionally, they were able to redirect 25% of their staff to other activities." The paper notes that "Quality culture improvements, which can include practices such as advanced preventive maintenance, enhanced employee training, and mature management of supplier quality, have been linked to higher performance."
Digital-twin scheduling unlocks lab capacity. A high-performing quality-control laboratory overlaid a "digital twin" on its daily sequencing logic. According to the FDA: "A company that was already performing well further improved its manufacturing labs' productivity by 40 to 50% percent through schedule optimization via an advanced-analytics digital-twin solution." This was achieved without adding headcount—purely through optimized resource utilization.
Lean Six Sigma pays off in a crisis. During a COVID-19 demand spike, the paper describes how "a pharmaceutical manufacturer used a Lean Six Sigma approach to identify and mitigate a tablet feed issue." The manufacturer improved multiple operational metrics and generated almost half a million dollars in savings, with additional projected savings for implementing this change at sister sites. Beyond the financial gains, "this initiative ultimately helped the manufacturer efficiently deliver medications to patients during a time of increased demand."
The iceberg you can’t afford to hit
The FDA visualizes the direct, indirect, and intangible costs of poor quality across manufacturers, patients, health-care systems, and society—most of them hidden "below the waterline."
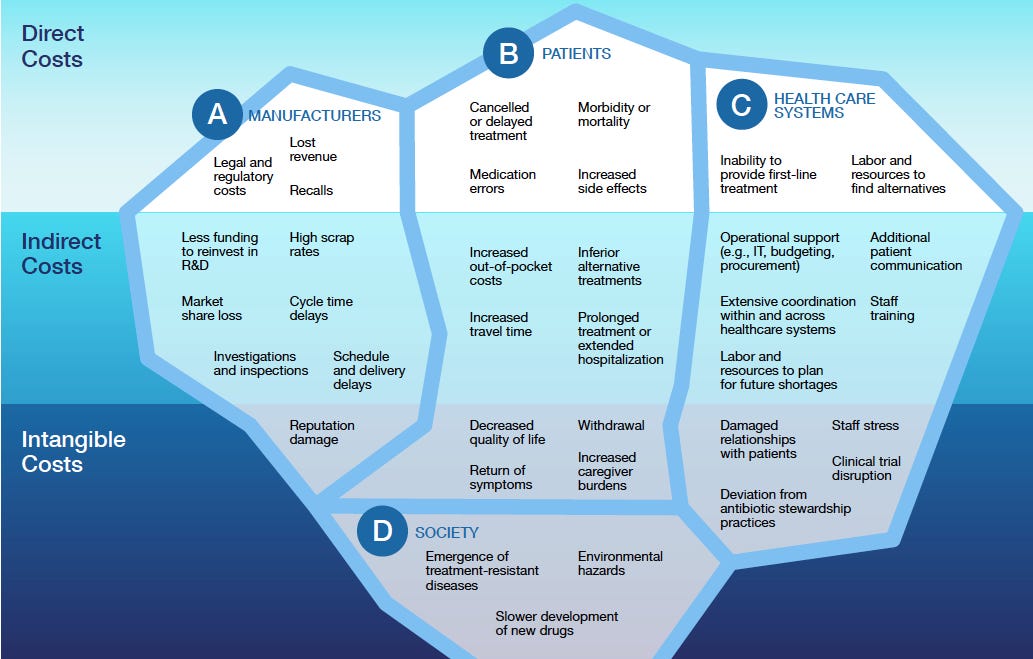
For manufacturers:
Direct costs: Recalls, lost revenue, legal and regulatory costs (the paper notes "poor quality management practices have caused billions of dollars in lost revenue for the pharmaceutical industry over the past two decades")
Indirect costs: High scrap rates, cycle time delays, market share loss, investigations and inspections
Intangible costs: Reputation damage that can affect stock market performance
For patients:
Direct costs: Cancelled or delayed treatment, medication errors, morbidity or mortality
Indirect costs: Increased out-of-pocket costs, prolonged treatment, inferior alternative treatments
Intangible costs: Decreased quality of life, return of symptoms, increased caregiver burdens
For healthcare systems:
Direct costs: Inability to provide first-line treatment, labor and resources to find alternatives
Indirect costs: Staff training, extensive coordination, operational support (IT, budgeting, procurement)
Intangible costs: Damaged relationships with patients, staff stress, clinical trial disruption
The paper emphasizes that estimates for the annual cost of labor to manage drug shortages in the United States range from $216-359 million, though these are "likely underestimates due to the lack of systematic data collection."
A trillion-dollar upside for public health
Inefficient manufacturing drains resources that could fund innovation. FDA calculates that "A 30% increase in manufacturing efficiency could potentially generate $1-12.3 trillion in social value through reinvestment of savings into research and development for new treatments."
The paper also reveals that the FDA analyzed the vision and mission statements of the top 50 health and pharmaceutical companies, finding that all emphasize public health, with "health" being the most frequently used word (32 mentions) compared to "business" (only 9 mentions).
The shortage connection
The white paper also makes a critical link between quality management and drug shortages.
"We know that about two thirds of medicine supply chain challenges begin as a quality issue. Shortages of medicines only exacerbate the quality issues that have been there all along."
This connection ties directly to the FDA's Quality Management Maturity (QMM) program, which the paper notes has been in development since 2019, when an interagency task force proposed creating a measurement and rating system based on objective indicators.
In April 2025, the FDA announced it is continuing its voluntary QMM prototype program for selected companies.
A few ways to use this paper
After reading this paper, we thought of several ways teams can operationalize or apply in their own work:
Locate yourself on the curve. The obvious first step here. Use the FDA's four-scenario model to benchmark where your QMS sits today and quantify the gap to "optimal." The paper provides clear characteristics for each level, from high error rates and rework at minimal investment to maximum efficiency at optimal investment.
Prioritize culture as infrastructure. Early wins, from preventive maintenance programs to supplier-quality dashboards, fund longer-term tech investments. The FDA specifically notes that even "suboptimal investment in quality management initiatives, companies can gain returns on investment through decreased costs and increased performance."
Target one high-impact process with data-driven tools. The paper's most dramatic ROI stories start with tightly scoped Lean Six Sigma or digital-twin pilots that can then be scaled across facilities.
Tie quality metrics to shortage-risk analytics. The FDA's public-health framing makes clear that resilience is now a board-level KPI, not a side benefit. The paper emphasizes that "mature quality management practices can increase companies' stability and reliability."
Plan for continuous, not one-off, investment. Over-investment is possible but unlikely; the real risk is stagnating at "sub-optimal." The FDA notes that companies can "strategically augment their quality management practices in stages that align with their business model and competitive situation."
Need help getting your QMS to “optimal” status?
The FDA Group’s bench of ex-FDA and industry SMEs can accelerate your journey to “optimal” by delivering:
Rapid maturity mapping against the FDA’s cost curve.
Hands-on remediation teams to close compliance and performance gaps.
Deployments that replicate the case-study wins above.
Reg-intel and shortage-risk advisory to turn quality data into supply-chain resilience.
Turn quality from a cost centre into a growth engine—partner with The FDA Group and start compounding ROI today.
Read one of our recent case studies for a look at how we helped stand up a quality system and provide ongoing support for a virtual pharma company.
FDA’s latest white paper doesn’t just urge better quality—it quantifies why it pays. The numbers are compelling; the public-health stakes are higher still. For firms willing to invest methodically, the path to value (and patient trust) has never been clearer.
As the FDA concludes:
“With the return on investment for both companies and public health, investing in quality management initiatives is an opportunity that pharmaceutical companies cannot afford to lose."
Who is The FDA Group?
The FDA Group helps life science organizations rapidly access the industry's best consultants, contractors, and candidates. Our resources assist in every stage of the product lifecycle, from clinical development to commercialization, with a focus on Quality Assurance, Regulatory Affairs, and Clinical Operations.
With thousands of resources worldwide, hundreds of whom are former FDA, we meet your precise resourcing needs through a fast, convenient talent selection process supported by a Total Quality Guarantee. Learn more and schedule a call with us to see if we’re a fit to help you access specialized professionals and execute your projects on time and on budget.
The FDA Group's Insider Newsletter is a reader-supported publication. To receive new posts and support our work, consider becoming a paid subscriber.




