FDA Issues Technical Amendments to Align Device Regulations With the New QMSR, Effective February 2, 2026
Regulatory housekeeping with strategic implications: the FDA just updated 179 sections across 21 CFR to reflect the QMSR shift. February 2026 isn’t far!
Only paid subscribers get regular full access to our guidance breakdowns and other analyses. If you’re not already a paid subscriber, you can upgrade here.
Device and medtech professionals, this is an important one for you.
The FDA has published a new final rule updating references across 21 CFR Parts 801, 803, 812, 860, 862, 864, 866, 868, 872, 874, 876, 878, 880, 882, 886, 888, 890, and 892 to align the broader medical device regulatory framework with the previously finalized Quality Management System Regulation (QMSR).
To be clear, this rule is technical, not substantive. The FDA emphasizes that it does not introduce new compliance obligations. Instead, it updates terminology, corrects past inconsistencies, and aligns outdated references to ensure regulatory coherence as the QMSR replaces the legacy Quality System Regulation (QSR).
Both the QMSR and these technical amendments become effective February 2, 2026. If you still need QMSR compliance support before February, get in touch to schedule your gap assessment and update project. We’re booking subject matter experts to work on QMSR updates through the end of the year and into 2026.
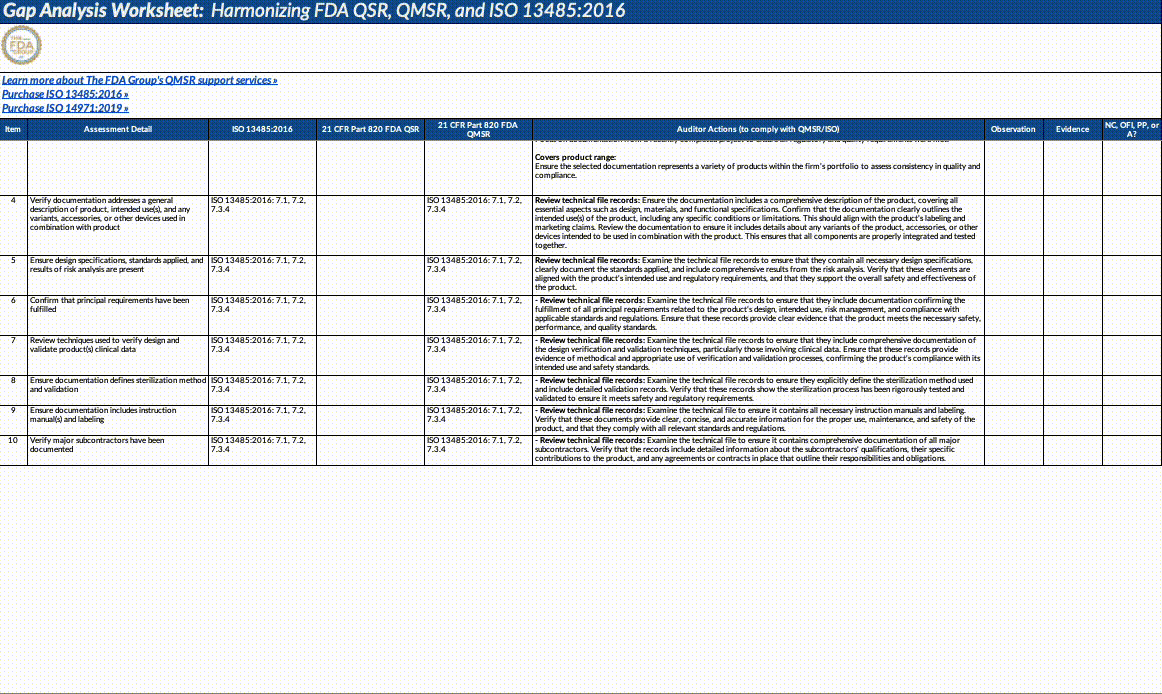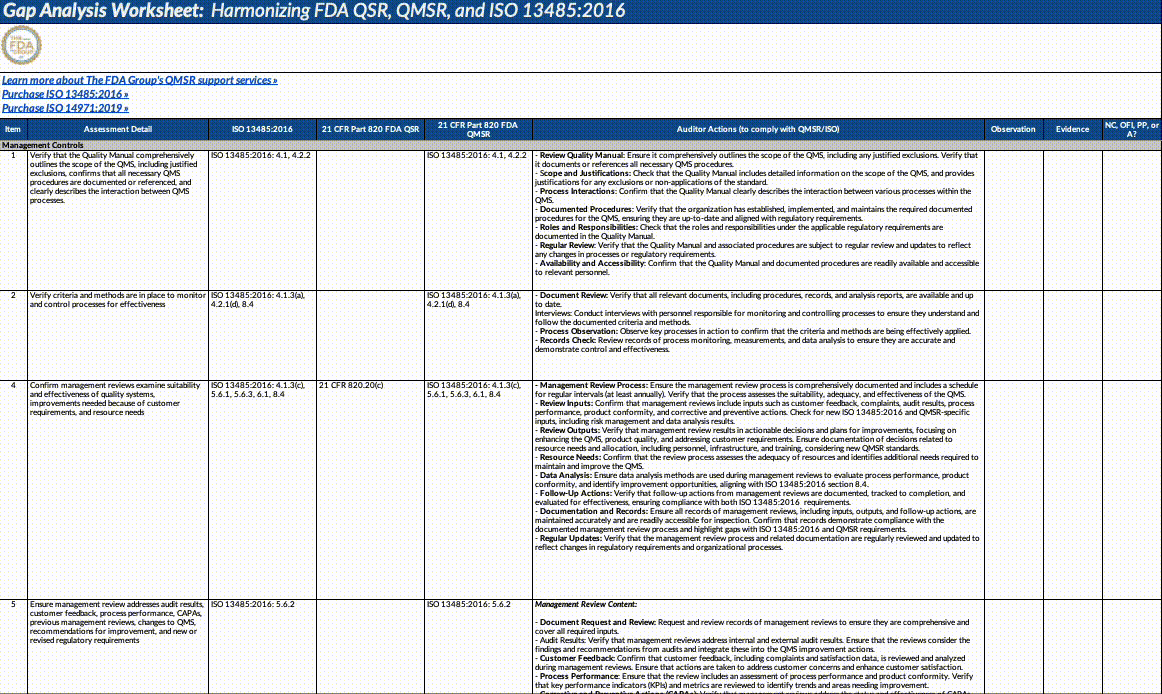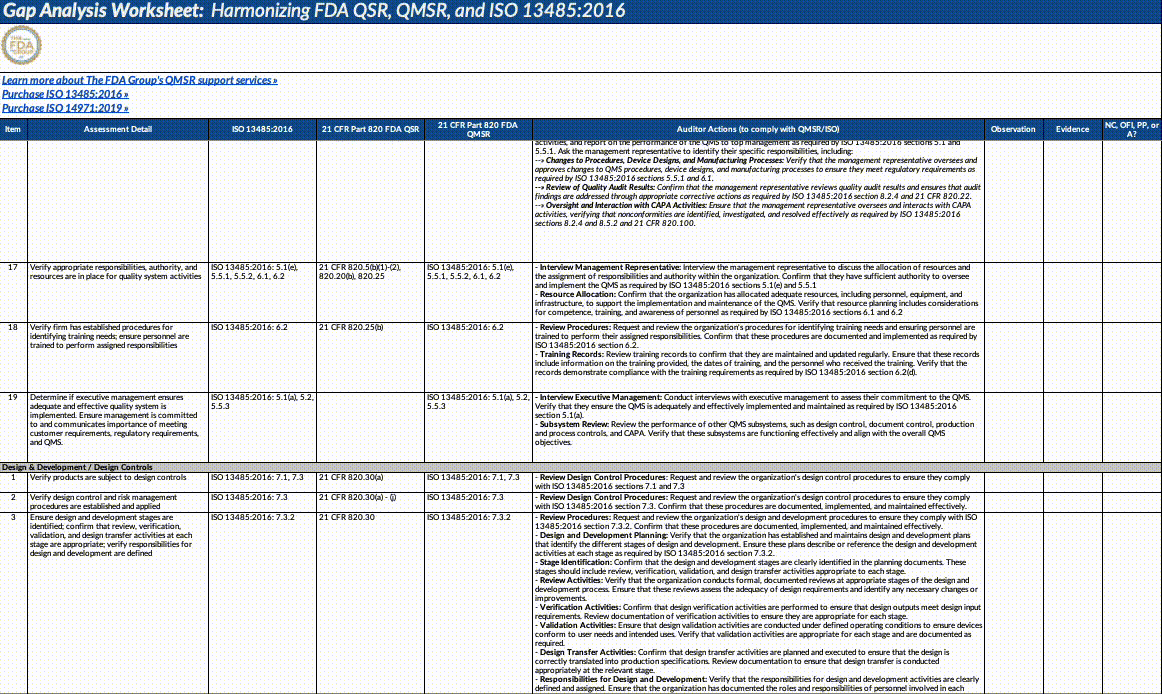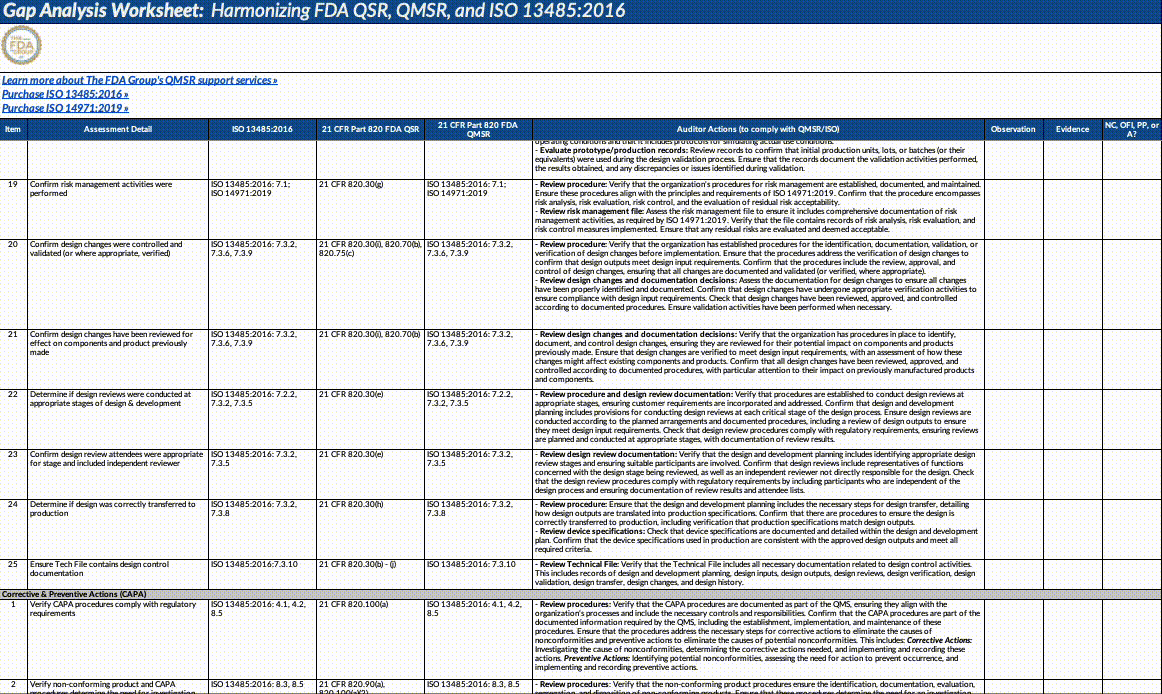
Also, paid subscribers can download our QMSR gap analysis worksheet here:
Here’s a distillation of the new guidance and what we recommend teams do in light of it.
Why this matters
While the amendment itself doesn’t impose new requirements, it reflects the agency’s final cleanup pass ahead of the QMSR transition. It replaces aging references to the former 21 CFR 820 QSR structure with references to the new ISO-aligned QMSR framework and ensures supporting regulations are internally consistent.
If your organization has already begun aligning with ISO 13485 and the QMSR Final Rule, this announcement will feel like confirmation, not change.
If you haven’t begun, this is the alarm bell!
What the FDA updated
The rule specifically modifies 179 individual regulatory sections, spanning device labeling, IDEs, medical device reporting, device classification rules, and dozens of classification entries.
Updates fall into the following major categories that we’ve outlined below (this is not an exhaustive list!). We strongly suggest opening the document itself and using our summary below as a guideline for reading through it and planning what to operationalize.
Again, if you need expert support here, talk to us. We have a deep bench of regulatory professionals who are helping firms transition into QMSR compliance, and deadlines are approaching.
1. Replacing QSR references with QMSR references
Many sections previously referenced QSR citations such as:
§ 820.180 (Records)
§ 820.198 (Complaint Files)
§ 820.30 (Design Controls)
This change appears most frequently in Class I exemptions and in multiple Class II classification regulations.
2. Updated terminology
Four regulatory sections now replace the phrase:
“quality system regulation”
with:
“quality management system regulation”
This change may appear small, but it reflects the formal retirement of the historical QSR vocabulary.
3. UDI and filing language alignment
Some regulatory passages update file terminology to reflect ISO-aligned wording. For example, references to the design history file now point to design and development files under the QMSR.
4. Fixing missing or incorrect text
A small number of corrections restore or clarify previously omitted regulatory language, most notably the missing device classification statement in § 886.1350 (keratoscope).
5. Formatting corrections
Several authority citations now correctly italicize portions of 21 U.S.C. 360l to avoid confusion with the numeral “1.”
What this does not do
FDA is explicit in stating:
No new regulatory requirements
No change in compliance expectations beyond the QMSR
No need for additional interpretation or rulemaking
According to the notice, these changes are “editorial in nature… and should not be construed as modifying any substantive standards or requirements.”
Because the changes are considered minor and administrative, the FDA issued the rule without public comment, using the Administrative Procedure Act good-cause exemption.
What to do now
Although this amendment doesn’t require corrective action, it reinforces a broader truth:
The era of the QSR is over. ISO-aligned QMS is now the U.S. regulatory baseline.
For teams preparing for the February 2026 deadline, this moment is a good checkpoint:
Is your QMS aligned with ISO 13485:2016 language and structure?
Have design and development records been mapped to the new requirements
Have internal audit programs been updated to the QMSR framework?
Have regulatory references in SOPs and templates been revised?
If the answer to any line above is a nervous “not yet,” now is the right time to begin remediation!
The FDA Group works with device manufacturers to:
Map legacy QSR systems to QMSR and ISO 13485:2016.
Update documentation, records architecture, and training.
Prepare teams for inspection changes under the new framework.
Learn more about our QMSR services.
Who is The FDA Group?
The FDA Group helps life science organizations rapidly access the industry's best consultants, contractors, and candidates. Our resources assist in every stage of the product lifecycle, from clinical development to commercialization, with a focus on Quality Assurance, Regulatory Affairs, and Clinical Operations.
With thousands of resources worldwide, hundreds of whom are former FDA, we meet your precise resourcing needs through a fast, convenient talent selection process supported by a Total Quality Guarantee. Learn more and schedule a call with us to see if we’re a fit to help you access specialized professionals and execute your projects on time and on budget.
The FDA Group's Insider Newsletter is a reader-supported publication. To receive new posts and support our work, consider becoming a paid subscriber.