Tracking Major Changes at the FDA: Your 3-Minute Explainer
Let's catch up on the news—there's a lot going on.
Editor’s Note (3/27/2025): In light of HHS’s newly announced reorganization plan, we’ve added a summary of the details at the top of this article.
The FDA is undergoing a pretty stark transformation under the new administration, with changes affecting staff, operations, and regulatory processes.
This special issue breaks down the most important developments and their potential impact on industry stakeholders.
Let’s jump right into it:
HHS announces 10k job cuts under Kennedy's reorg plan
THS announced Thursday it will cut 10,000 full-time positions as part of Secretary Robert F. Kennedy Jr.'s "Make America Healthy Again" reorganization.
These cuts, combined with 10,000 employees who have already voluntarily departed, will reduce HHS staffing from 82,000 to 62,000 full-time employees, according to the department.
The government projects annual savings of approximately $1.8 billion from these reductions.
According to an HHS fact sheet, the cuts will significantly impact several agencies:
FDA: 3,500 workers (19% of staff), focusing on "streamlining operations and centralizing administrative functions." HHS stated the reduction "will not affect drug, medical device, or food reviewers, nor will it impact inspectors."
CDC: 2,400 workers (18% of staff), though the net decrease would be 1,400 when accounting for approximately 1,000 individuals transferring from ASPR.
NIH: 1,200 workers (6% of staff) through centralization of "procurement, human resources, and communications across its 27 institutes and centers."
CMS: 300 workers, with emphasis on reducing duplication.
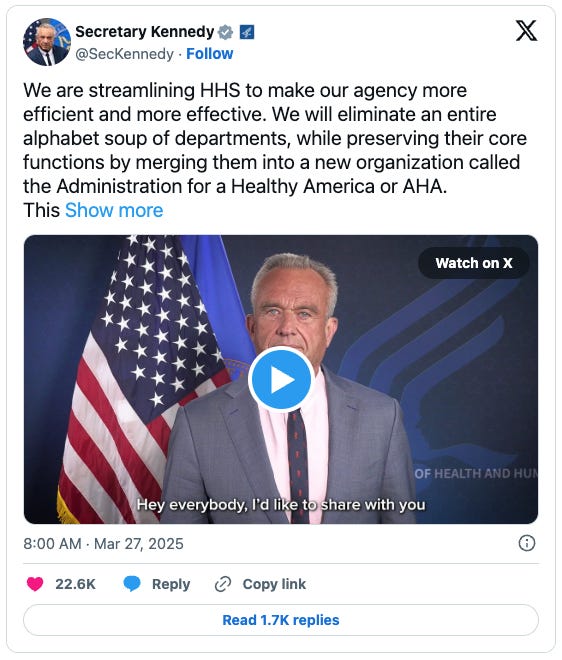
The reorganization will create a new Administration for a Healthy America, consolidating multiple agencies, including the Office of the Assistant Secretary for Health, Health Resources and Services Administration, Substance Abuse and Mental Health Services Administration, Agency for Toxic Substances and Disease Registry, and National Institute for Occupational Safety and Health.
Additionally, the Administration for Strategic Preparedness and Response, responsible for disaster and public health emergency response, will be transferred to the CDC.
"We will eliminate an entire alphabet soup of departments, while preserving their core functions by merging them into a new organization called the Administration for a Healthy America," Kennedy said in a statement.
Despite exemptions for FDA inspectors and reviewers, we anticipate that the downsizing could still affect drug and device review timelines.
Read the full HHS statement, and see other reporting from CBS News, NBC Los Angeles, MedTech Dive, and AOL.
Makary confirmed
The Senate on Tuesday night confirmed Marty Makary to be the Food and Drug Administration commissioner. Makary was easily confirmed by a 56-44 vote, picking up three Democrats.
During his confirmation hearings, he provided limited insight into his policy priorities. One clear position he expressed was support for increasing over-the-counter product availability, citing Narcan and blood glucose monitors (though both were already available OTC).
Mandatory return to office creates logistical challenges for staff
FDA staff returned to the White Oak campus on March 17th, following the administration's elimination of remote work for federal employees. The transition has created several logistical challenges:
Security lines stretching to over an hour at the first checkpoint, with additional time needed to clear the second security checkpoint.
Severe parking shortages require the agency to lease 1,300 additional spots in downtown Silver Spring with shuttle service to campus.
Insufficient office space, with the agency resorting to folding tables and auditorium chairs to accommodate thousands of returning workers.
Limited equipment, such as docking stations, in newly created work areas, with staff reporting increased noise and disruption.
These challenges are compounded by the fact that the White Oak campus was not designed to accommodate the full FDA workforce simultaneously, an issue experts had warned about before the return-to-office mandate was implemented.
Workforce reduction initiatives are in full swing
Multiple efforts are underway to reduce the FDA's workforce. A "RIF" (reduction in force) has affected FDA staffing levels Some positions, particularly in CDRH, were initially included in layoffs but later "called back" after industry pushback HHS Secretary Robert F. Kennedy Jr. appears to be leading these reduction efforts, with FDA leadership having "more of a supporting role."
Beyond direct layoffs, an "attrition plan" has been implemented that requires the FDA to hire no more than one employee for every four that depart; creates what can be called intentionally unfavorable work environment to encourage departures, and includes buyout offers ($25,000 + 8 weeks to find new employment) that many senior FDA staff are considering.
More details:
Voluntary Separation Incentive Payments (VSIP): A $25,000 buyout offered to eligible staff who voluntarily leave by March 14th. Notably, this does not apply to review staff in medical product centers, inspectors, investigators, or Public Health Service Commissioned Corps officers. Employees accepting the buyout receive eight weeks of administrative leave with full pay and benefits. (More in RAPS.)
Potential Demotions: During a virtual information session with 8,000 staff, the agency discussed potential "reasonable offers" involving demotions of up to two General Schedule (GS) levels. Staff who refuse these demotions would waive their rights to future severance packages, a prospect one staffer described as "laughable" and likely to trigger mass resignations. (More in RAPS.)
Probationary Workers: Workers terminated in February have been temporarily reinstated with paid administrative leave following a temporary restraining order issued by US District Judge James Bredar, who ruled the administration illegally instituted mass layoffs without providing workers advanced notice. (More in RAPS.)
Communications and external engagement severely restricted
FDA staff face significant limitations on external communications following executive orders from President Trump:
Travel restrictions are preventing in-person attendance at key global regulatory forums, including the International Medical Device Regulators Forum (IMDRF) spring meeting in Tokyo. (More in RAPS.)